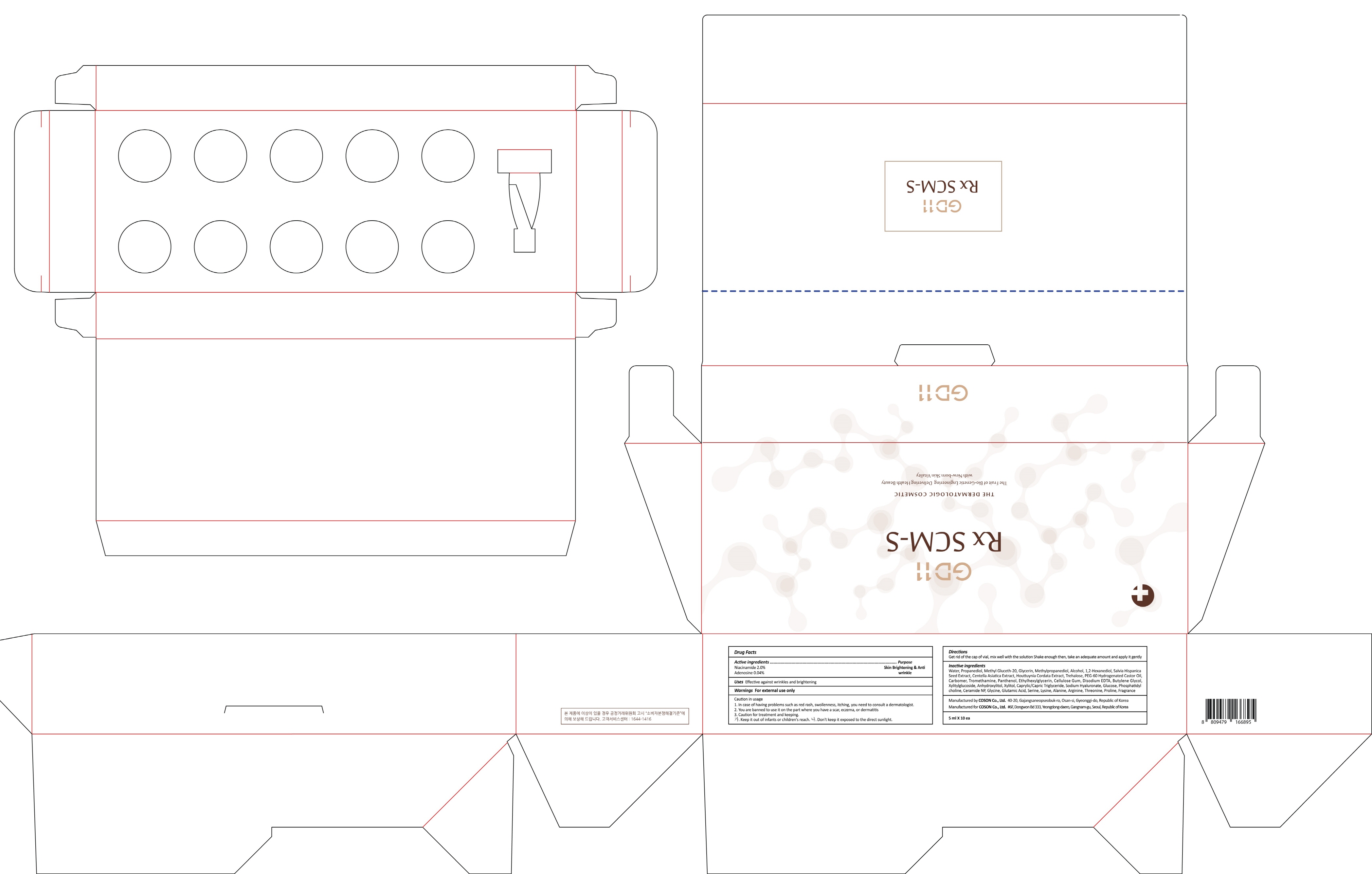
GD11 RX SCM S- niacinamide, adenosine liquid
GD11 Rx SCM S by
Drug Labeling and Warnings
GD11 Rx SCM S by is a Otc medication manufactured, distributed, or labeled by Coson Co., Ltd., COSON Co., Ltd._Osan Plant. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Inactive ingredients: Water, Propanediol, Methyl Gluceth-20, Glycerin, Methylpropanediol, Alcohol, 1,2-Hexanediol, Salvia Hispanica Seed Extract, Centella Asiatica Extract, Houttuynia Cordata Extract, Trehalose, PEG-60 Hydrogenated Castor Oil, Carbomer, Tromethamine, Panthenol, Ethylhexylglycerin, Cellulose Gum, Disodium EDTA, Butylene Glycol, Xylitylglucoside, Anhydroxylitol, Xylitol, Caprylic/Capric Triglyceride, Sodium Hyaluronate, Glucose, Phosphatidylcholine, Ceramide NP, Glycine, Glutamic Acid, Serine, Lysine, Alanine, Arginine, Threonine, Proline, Fragrance
- PURPOSE
-
WARNINGS
Warnings: For external use only
Caution in usage 1. In case of having problems such as red rash, swollenness, itching, you need to consult a dermatologist. 2. You are banned to use it on the part where you have a scar, eczema, or dermatitis 3. Caution for treatment and keeping. - Keep it out of infants or children's reach. - Don't keep it exposed to the direct sunlight
- KEEP OUT OF REACH OF CHILDREN
- Uses
- Directions
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GD11 RX SCM S
niacinamide, adenosine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 62171-090 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Niacinamide (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) Niacinamide 0.10 g in 5 mL Adenosine (UNII: K72T3FS567) (ADENOSINE - UNII:K72T3FS567) Adenosine 0.002 g in 5 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Propanediol (UNII: 5965N8W85T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62171-090-02 10 in 1 CARTON 07/01/2017 1 NDC: 62171-090-01 5 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/01/2017 Labeler - Coson Co., Ltd. (689835593) Registrant - Coson Co., Ltd. (689835593) Establishment Name Address ID/FEI Business Operations COSON Co., Ltd._Osan Plant 689847210 manufacture(62171-090)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.