ICLUSIG- ponatinib hydrochloride tablet, film coated
Iclusig by
Drug Labeling and Warnings
Iclusig by is a Prescription medication manufactured, distributed, or labeled by Takeda Pharmaceuticals America, Inc., Ash Stevens LLC, Catalent Micron Technologies, Inc., Catalent Pharma Solutions, LLC, Sharp Packaging Services, LLC, Patheon Inc., Takeda Ireland Limited, Almac Sciences (Ireland) Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ICLUSIG safely and effectively. See full prescribing information for ICLUSIG.
ICLUSIG® (ponatinib) tablets, for oral use
Initial U.S. Approval: 2012WARNING: ARTERIAL OCCLUSION, VENOUS THROMBOEMBOLISM, HEART FAILURE, and HEPATOTOXICITY
See full prescribing information for complete boxed warning.
- Arterial occlusion has occurred in at least 35% of Iclusig-treated patients including fatal myocardial infarction, stroke, stenosis of large arterial vessels of the brain, severe peripheral vascular disease, and the need for urgent revascularization procedures. Patients with and without cardiovascular risk factors, including patients less than 50 years old, experienced these events. Interrupt or stop Iclusig immediately for arterial occlusion. A benefit-risk consideration should guide a decision to restart Iclusig (5.1).
- Venous thromboembolism has occurred in 6% of Iclusig-treated patients. Monitor for evidence of thromboembolism. Consider dose modification or discontinuation of Iclusig in patients who develop serious venous thromboembolism (5.2).
- Heart failure, including fatalities, occurred in 9% of Iclusig-treated patients. Monitor cardiac function. Interrupt or stop Iclusig for new or worsening heart failure (5.3).
- Hepatotoxicity, liver failure and death have occurred in Iclusig-treated patients. Monitor hepatic function. Interrupt Iclusig if hepatotoxicity is suspected (2.3, 5.4).
RECENT MAJOR CHANGES
Warnings and Precautions Impaired Wound Healing and Gastrointestinal Perforation (5.16) 1/2020 INDICATIONS AND USAGE
Iclusig is a kinase inhibitor indicated for the:
- Treatment of adult patients with chronic phase, accelerated phase, or blast phase chronic myeloid leukemia (CML) or Ph+ ALL for whom no other tyrosine kinase inhibitor (TKI) therapy is indicated. (1)
- Treatment of adult patients with T315I-positive CML (chronic phase, accelerated phase, or blast phase) or T315I-positive Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ ALL). (1)
Limitations of Use:
Iclusig is not indicated and is not recommended for the treatment of patients with newly diagnosed chronic phase CML. (5.7)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Tablets: 15 mg, 30 mg and 45 mg (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Hypertension: Monitor for high blood pressure and manage as clinically indicated. (5.5)
- Pancreatitis: Monitor serum lipase monthly; interrupt or discontinue Iclusig. (2.3, 5.6)
- Neuropathy: Monitor for symptoms of peripheral and cranial neuropathy. (5.8)
- Ocular Toxicity: Conduct comprehensive eye exams at baseline and periodically during treatment. (5.9)
- Hemorrhage: Interrupt Iclusig for serious or severe hemorrhage. (5.10)
- Fluid Retention: Monitor patients for fluid retention; interrupt, reduce, or discontinue Iclusig. (5.11)
- Cardiac Arrhythmias: Monitor for symptoms of arrhythmias. (5.12, 6)
- Myelosuppression: Thrombocytopenia, neutropenia, and anemia may require dose interruption or reduction. Monitor complete blood counts every 2 weeks for 3 months and then monthly and as clinically indicated. Interrupt Iclusig for ANC <1000/mm3 or thrombocytopenia <50,000/mm3. (2.2, 5.13)
- Tumor Lysis Syndrome: Ensure adequate hydration and correct elevated uric acid levels prior to initiating therapy with Iclusig. (5.14)
- Reversible Posterior Leukoencephalopathy Syndrome (RPLS): Interrupt Iclusig and resume treatment only once the event is resolved and if the benefit of continued treatment outweighs the risk of RPLS. (5.15)
- Impaired Wound Healing and Gastrointestinal Perforation: Withhold Iclusig for at least 1 week prior to elective surgery. Do not administer for at least 2 weeks following major surgery and until adequate wound healing. The safety of resumption of Iclusig after resolution of wound healing complications has not been established. (5.16)
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception. (5.17, 8.1, 8.3)
ADVERSE REACTIONS
The most common non-hematologic adverse reactions (≥20%) were, abdominal pain, rash, constipation, headache, dry skin, arterial occlusion, fatigue, hypertension, pyrexia, arthralgia, nausea, diarrhea, lipase increased, vomiting, myalgia and pain in extremity. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceutical Co. Ltd. at 1-844-817-6468 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 1/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: ARTERIAL OCCLUSION, VENOUS THROMBOEMBOLISM, HEART FAILURE, and HEPATOTOXICITY
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dose Modifications for Myelosuppression
2.3 Dose Modifications for Non-hematologic Adverse Reactions
2.4 Dose Modification for Use with Strong CYP3A Inhibitors
2.5 Dose Modification for Use in Patients with Hepatic Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Arterial Occlusion
5.2 Venous Thromboembolism
5.3 Heart Failure
5.4 Hepatotoxicity
5.5 Hypertension
5.6 Pancreatitis
5.7 Increased Toxicity in Newly Diagnosed Chronic Phase CML
5.8 Neuropathy
5.9 Ocular Toxicity
5.10 Hemorrhage
5.11 Fluid Retention
5.12 Cardiac Arrhythmias
5.13 Myelosuppression
5.14 Tumor Lysis Syndrome
5.15 Reversible Posterior Leukoencephalopathy Syndrome (RPLS)
5.16 Impaired Wound Healing and Gastrointestinal Perforation
5.17 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drugs that are Strong Inhibitors of CYP3A Enzymes
7.2 Drugs that are Strong Inducers of CYP3A Enzymes
7.3 Drugs that Elevate Gastric pH
7.4 Drugs that are Substrates of the P-gp or ABCG2 Transporter Systems
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: ARTERIAL OCCLUSION, VENOUS THROMBOEMBOLISM, HEART FAILURE, and HEPATOTOXICITY
Arterial Occlusion:
- Arterial occlusions have occurred in at least 35% of Iclusig-treated patients. Some patients experienced more than 1 type of event. Events observed included fatal myocardial infarction, stroke, stenosis of large arterial vessels of the brain, severe peripheral vascular disease, and the need for urgent revascularization procedures. Patients with and without cardiovascular risk factors, including patients age 50 years or younger, experienced these events. Monitor for evidence of arterial occlusion. Interrupt or stop Iclusig immediately for arterial occlusion. A benefit-risk consideration should guide a decision to restart Iclusig therapy (5.1).
Venous Thromboembolism:
- Venous occlusive events have occurred in 6% of Iclusig-treated patients. Monitor for evidence of venous thromboembolism. Consider dose modification or discontinuation of Iclusig in patients who develop serious venous thromboembolism (5.2).
Heart Failure:
- Heart failure, including fatalities, occurred in 9% of Iclusig-treated patients. Monitor cardiac function. Interrupt or stop Iclusig for new or worsening heart failure (5.3).
-
1 INDICATIONS AND USAGE
Iclusig (ponatinib) is a kinase inhibitor indicated for the:
- Treatment of adult patients with chronic phase, accelerated phase, or blast phase chronic myeloid leukemia (CML) or Ph+ ALL for whom no other tyrosine kinase inhibitor (TKI) therapy is indicated.
- Treatment of adult patients with T315I-positive CML (chronic phase, accelerated phase, or blast phase) or T315I-positive Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ ALL).
Limitations of Use:
Iclusig is not indicated and is not recommended for the treatment of patients with newly diagnosed chronic phase CML [see Warnings and Precautions (5.7)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The optimal dose of Iclusig has not been identified. In clinical trials, the starting dose of Iclusig was 45 mg administered orally once daily. However, in the Phase 2 trial, 68% of the patients required dose reductions to 30 mg or 15 mg once daily during the course of therapy.
Start dosing with 45 mg once daily. Consider reducing the dose of Iclusig for patients with chronic phase (CP) CML and accelerated phase (AP) CML who have achieved a major cytogenetic response.
Consider discontinuing Iclusig if response has not occurred by 3 months (90 days).
Iclusig may be taken with or without food. Tablets should be swallowed whole.
2.2 Dose Modifications for Myelosuppression
Suggested dose modifications for neutropenia (ANC* less than 1.0 × 109/L) and thrombocytopenia (platelet less than 50 × 109/L) that are unrelated to leukemia are summarized in Table 1.
Table 1: Suggested Dose Modifications for Myelosuppression - * ANC = absolute neutrophil count
ANC* <1 × 109/L
or
platelet <50 × 109/LFirst occurrence: - Interrupt Iclusig and resume initial 45 mg dose after recovery to ANC ≥1.5 × 109/L and platelet ≥75 × 109/L
Second occurrence: - Interrupt Iclusig and resume at 30 mg after recovery to ANC ≥1.5 × 109/L and platelet ≥75 × 109/L
Third occurrence: - Interrupt Iclusig and resume at 15 mg after recovery to ANC ≥1.5 × 109/L and platelet ≥75 × 109/L
2.3 Dose Modifications for Non-hematologic Adverse Reactions
If a serious non-hematologic adverse reaction occurs, modify the dose, interrupt treatment, or consider discontinuation. Do not restart Iclusig in patients with arterial or venous occlusive reactions unless the potential benefit outweighs the risk of recurrent arterial or venous occlusions and the patient has no other treatment options. For serious reactions other than arterial or venous occlusion, do not restart Iclusig until the serious event has resolved or the potential benefit of resuming therapy is judged to outweigh the risk.
Hepatotoxicity
Recommended modifications for hepatotoxicity are summarized in Table 2.
Table 2: Recommended Dose Modifications for Hepatotoxicity - * ULN = Upper Limit of Normal for the lab
Elevation of liver transaminase >3× ULN* (Grade 2 or higher) Occurrence at 45 mg: - Interrupt Iclusig and monitor hepatic function
- Resume Iclusig at 30 mg after recovery to ≤ Grade 1 (<3× ULN)
- Interrupt Iclusig and resume at 15 mg after recovery to ≤ Grade 1
- Discontinue Iclusig
Elevation of AST or ALT ≥3× ULN concurrent with an elevation of bilirubin >2× ULN and alkaline phosphatase <2× ULN Discontinue Iclusig Pancreatitis and Elevation of Lipase
Recommended modifications for pancreatic adverse reactions are summarized in Table 3.
Table 3: Recommended Dose Modifications for Pancreatitis and Elevation of Lipase - * ULN = Upper Limit of Normal for the lab
Asymptomatic Grade 1 or 2 elevation of serum lipase Consider interruption or dose reduction of Iclusig Asymptomatic Grade 3 or 4 elevation of lipase (>2× ULN*) or asymptomatic radiologic pancreatitis (Grade 2 pancreatitis) Occurrence at 45 mg: - Interrupt Iclusig and resume at 30 mg after recovery to ≤ Grade 1 (<1.5× ULN)
- Interrupt Iclusig and resume at 15 mg after recovery to ≤ Grade 1
- Discontinue Iclusig
Symptomatic Grade 3 pancreatitis Occurrence at 45 mg: - Interrupt Iclusig and resume at 30 mg after complete resolution of symptoms and after recovery of lipase elevation to ≤ Grade 1
- Interrupt Iclusig and resume at 15 mg after complete resolution of symptoms and after recovery of lipase elevation to ≤ Grade 1
- Discontinue Iclusig
Grade 4 pancreatitis Discontinue Iclusig 2.4 Dose Modification for Use with Strong CYP3A Inhibitors
The recommended dose should be reduced to 30 mg once daily when administering Iclusig with strong CYP3A inhibitors [see Drug Interactions (7.1)].
2.5 Dose Modification for Use in Patients with Hepatic Impairment
The recommended starting dose is 30 mg once daily in patients with hepatic impairment (Child-Pugh A, B, or C) [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Arterial Occlusion
Arterial occlusions, including fatal myocardial infarction, stroke, stenosis of large arterial vessels of the brain, severe peripheral vascular disease have occurred in at least 35% of Iclusig-treated patients from the Phase 1 and Phase 2 trials. With a minimum of 48 months follow-up for ongoing patients (N=133) in the Phase 2 trial, 33% (150/449) of Iclusig-treated patients experienced a cardiac vascular (21%), peripheral vascular (12%), or cerebrovascular (9%) arterial occlusive event; some patients experienced more than 1 type of arterial occlusive event.
Iclusig can cause fatal and life-threatening arterial occlusion within 2 weeks of starting treatment, and at dose levels as low as 15 mg per day. Iclusig can also cause recurrent or multisite vascular occlusion. Patients have required revascularization procedures (coronary, cerebrovascular, and peripheral arterial).
In the Phase 2 trial, the median time to onset of the first cardiac vascular, cerebrovascular, and peripheral vascular arterial occlusive events was 193 (range: 1 to 1355), 526 (range: 5 to 1339), and 478 (range: 3 to 1344) days, respectively.
Patients with and without cardiovascular risk factors, including patients age 50 years or younger, experienced these events. The most common risk factors observed in patients with arterial occlusive events were hypertension (62%; 93/150), hyperlipidemia (61%; 91/150), and history of cardiac disease (48%; 72/150). Arterial occlusion adverse events were more frequent with increasing age and in patients with history of ischemia, hypertension, diabetes, or hyperlipidemia (see Table 4).
Table 4: Arterial Occlusion Incidence in Iclusig-Treated Patients in Phase 2 Trial According to Risk Categories: 4 year follow-up Age
(At time of study entry)History of ischemia, hypertension, diabetes, or hyperlipidemia
N=218No history of ischemia, hypertension, diabetes, or hyperlipidemia
N=23149 or younger 31%
(11/36)19%
(21/108)50 to 74 years 40%
(64/158)30%
(32/109)75 and older 58%
(14/24)57%
(8/14)All age groups 41%
(89/218)26%
(61/231)Total 33%
(150/449)Cardiac vascular occlusion, including fatal and life-threatening myocardial infarction and coronary artery occlusion has occurred in 21% (94/449) of Iclusig-treated patients. Patients have developed heart failure concurrent or subsequent to the myocardial ischemic event [see Warnings and Precautions (5.3)].
Cerebrovascular occlusion, including fatal stroke, has occurred in 9% (40/449) of Iclusig-treated patients. Iclusig can cause stenosis over multiple segments in major arterial vessels that supply the brain (e.g., carotid, vertebral, middle cerebral artery).
Peripheral arterial occlusion, including fatal mesenteric artery occlusion and life-threatening peripheral arterial disease, have occurred in 12% (52/449) of Iclusig-treated patients. Patients have developed digital or distal extremity necrosis and have required amputations. Renal artery stenosis, associated with worsening, labile or treatment-resistant hypertension, has occurred in some Iclusig-treated patients [see Warnings and Precautions (5.5)].
Clinicians should consider whether the benefits of Iclusig treatment are expected to exceed the risks of therapy. In patients suspected of developing arterial occlusive events, interrupt or stop Iclusig. A benefit-risk consideration should guide a decision to restart Iclusig therapy [see Dosage and Administration (2.3)].
5.2 Venous Thromboembolism
Venous thromboembolic events occurred in 6% (25/449) of Iclusig-treated patients, including deep venous thrombosis (10 patients), pulmonary embolism (7 patients), superficial thrombophlebitis (3 patients), and retinal vein thrombosis (2 patients) with vision loss.
In the Phase 2 trial, the incidence of venous thromboembolism was 9% (3/32) in patients with Ph+ ALL, 10% (6/62) in patients with blast phase (BP) CML, 4% (3/85) in patients with AP-CML, and 5% (13/270) in patients with CP-CML. Consider dose modification or discontinuation of Iclusig in patients who develop serious venous thromboembolism [see Dosage and Administration (2.3)].
5.3 Heart Failure
Fatal or serious heart failure or left ventricular dysfunction occurred in 6% of Iclusig-treated patients (N=29/449) in the Phase 2 trial (48 months follow-up). Nine percent of patients (N=39) experienced any grade of heart failure or left ventricular dysfunction. The most frequently reported heart failure events were congestive cardiac failure and decreased ejection fraction (in 14 patients each; 3%).
Monitor patients for signs or symptoms consistent with heart failure and treat as clinically indicated, including interruption of Iclusig. Consider discontinuation of Iclusig in patients who develop serious heart failure [see Dosage and Administration (2.3)].
5.4 Hepatotoxicity
Iclusig can cause hepatotoxicity, including liver failure and death. Fulminant hepatic failure leading to death occurred in an Iclusig-treated patient within 1 week of starting Iclusig. Two additional fatal cases of acute liver failure also occurred. The fatal cases occurred in patients with blast phase (BP) CML or Ph+ ALL. Severe (Grade 3 or 4) hepatotoxicity occurred in all disease cohorts.
With 48 months follow-up, 11% (50/449) of Iclusig-treated patients experienced Grade 3 or 4 hepatotoxicity in the Phase 2 trial. The most common forms of hepatotoxicity were elevations of aspartate aminotransferase (AST) or alanine aminotransferase (ALT), bilirubin, and alkaline phosphatase. The incidence of AST or ALT elevation was 54% (all Grades) and 8% (Grade 3 or 4). ALT or AST elevation was not reversed by the date of last follow-up in 5% of patients.
Hepatotoxic events were observed in 29% of patients. The median time to onset of hepatotoxicity event was 3 months, with a range of <1 month to 47 months. Monitor liver function tests at baseline, then at least monthly or as clinically indicated. Interrupt, reduce or discontinue Iclusig as clinically indicated [see Dosage and Administration (2.3)].
5.5 Hypertension
Treatment-emergent elevation of systolic or diastolic blood pressure (BP) occurred in 68% (306/449) of patients in the Phase 2 clinical trial (48 months of follow-up). Fifty-three patients (12%) treated with Iclusig in this clinical trial experienced treatment-emergent symptomatic hypertension as a serious adverse reaction, including hypertensive crisis. Patients may require urgent clinical intervention for hypertension associated with confusion, headache, chest pain, or shortness of breath [see Adverse Reactions (6)].
In patients with baseline systolic BP <140 mm Hg and baseline diastolic BP <90 mm Hg, 80% (229/285) experienced treatment-emergent hypertension; 44% (124/285) developed Stage 1 hypertension (defined as systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg) while 37% developed Stage 2 hypertension (defined as systolic BP ≥160 mm Hg or diastolic BP ≥100 mm Hg). In 132 patients with Stage 1 hypertension at baseline, 67% (88/132) developed Stage 2 hypertension.
Monitor and manage blood pressure elevations during Iclusig use and treat hypertension to normalize blood pressure. Interrupt, dose reduce, or stop Iclusig if hypertension is not medically controlled. In the event of significant worsening, labile or treatment-resistant hypertension, interrupt treatment and consider evaluating for renal artery stenosis.
5.6 Pancreatitis
Pancreatitis occurred in 7% (31/449, 6% serious or Grade 3/4) of Iclusig-treated patients with 48 months of follow-up in the Phase 2 trial. The incidence of treatment-emergent lipase elevation was 42% (16% Grade 3 or greater).
Pancreatitis resulted in discontinuation or treatment interruption in 6% of patients (26/449). The median time to onset of pancreatitis was 14 days (range: 3 to 1452). Twenty-three of the 31 cases of pancreatitis resolved within 2 weeks with dose interruption or reduction.
Check serum lipase every 2 weeks for the first 2 months and then monthly thereafter or as clinically indicated. Consider additional serum lipase monitoring in patients with a history of pancreatitis or alcohol abuse. Dose interruption or reduction may be required. In cases where lipase elevations are accompanied by abdominal symptoms, interrupt treatment with Iclusig and evaluate patients for pancreatitis [see Dosage and Administration (2.3)]. Do not consider restarting Iclusig until patients have complete resolution of symptoms and lipase levels are less than 1.5× ULN.
5.7 Increased Toxicity in Newly Diagnosed Chronic Phase CML
In a prospective randomized clinical trial in the first line treatment of newly diagnosed patients with chronic phase (CP) CML, single agent Iclusig 45 mg once daily increased the risk of serious adverse reactions 2-fold compared to single agent imatinib 400 mg once daily. The median exposure to treatment was less than 6 months. The trial was halted for safety in October 2013.
Arterial and venous thrombosis and occlusions occurred at least twice as frequently in the Iclusig arm compared to the imatinib arm. Compared to imatinib-treated patients, Iclusig-treated patients exhibited a greater incidence of myelosuppression, pancreatitis, hepatotoxicity, cardiac failure, hypertension, and skin and subcutaneous tissue disorders. Iclusig is not indicated and is not recommended for the treatment of patients with newly diagnosed CP-CML.
5.8 Neuropathy
Peripheral and cranial neuropathy have occurred in Iclusig-treated patients. Overall, 20% (90/449) of Iclusig-treated patients in the pivotal Phase 2 trial experienced a peripheral neuropathy event of any grade (2%, Grade 3/4) (48 months follow-up). The most common peripheral neuropathies reported were paresthesia (5%, 23/449), neuropathy peripheral (4%, 19/449), hypoesthesia (3%, 15/449), dysgeusia (2%, 10/449), muscular weakness (2%, 10/449) and hyperesthesia (1%, 5/449). Cranial neuropathy developed in 2% (10/449) of Iclusig-treated patients (<1%, 3/449 - Grade 3/4).
Of the patients who developed neuropathy, 26% (23/90) developed neuropathy during the first month of treatment. Monitor patients for symptoms of neuropathy, such as hypoesthesia, hyperesthesia, paresthesia, discomfort, a burning sensation, neuropathic pain or weakness. Consider interrupting Iclusig and evaluate if neuropathy is suspected.
5.9 Ocular Toxicity
Serious ocular toxicities leading to blindness or blurred vision have occurred in Iclusig-treated patients in the Phase 2 trial (48 months follow-up). Retinal toxicities including macular edema, retinal vein occlusion, and retinal hemorrhage occurred in 2% of Iclusig-treated patients. Conjunctival irritation, corneal erosion or abrasion, dry eye, conjunctivitis, conjunctival hemorrhage, hyperaemia and edema or eye pain occurred in 14% of patients. Visual blurring occurred in 6% of patients. Other ocular toxicities include cataracts, periorbital edema, blepharitis, glaucoma, eyelid edema, ocular hyperaemia, iritis, iridocyclitis, and ulcerative keratitis. Conduct comprehensive eye exams at baseline and periodically during treatment [see Adverse Reactions (6)].
5.10 Hemorrhage
Serious hemorrhage events including fatalities, occurred in 6% (28/449) of patients treated with Iclusig in the Phase 2 trial, with 48 months follow-up. Hemorrhage occurred in 28% (124/449) of patients. The incidence of serious bleeding events was higher in patients with AP-CML, BP-CML, and Ph+ ALL. Gastrointestinal hemorrhage and subdural hematoma were the most commonly reported serious bleeding events occurring in 1% (4/449 and 4/449, respectively). Most hemorrhagic events, but not all, occurred in patients with Grade 4 thrombocytopenia [see Warnings and Precautions (5.13)]. Interrupt Iclusig for serious or severe hemorrhage and evaluate [see Dosage and Administration (2.3)].
5.11 Fluid Retention
Fluid retention events judged as serious occurred in 4% (18/449) of patients treated with Iclusig in the Phase 2 trial (48 months follow-up). One instance of brain edema was fatal. For fluid retention events occurring in more than 2% of the patients (treatment-emergent), serious cases included: pleural effusion (7/449, 2%), pericardial effusion (4/449, 1%), and edema peripheral (2/449, <1%).
In total, fluid retention occurred in 31% of the patients. The most common fluid retention events were peripheral edema (17%), pleural effusion (8%), pericardial effusion (4%) and peripheral swelling (3%).
Monitor patients for fluid retention and manage patients as clinically indicated. Interrupt, reduce, or discontinue Iclusig as clinically indicated [see Dosage and Administration (2.3)].
5.12 Cardiac Arrhythmias
Arrhythmias occurred in 19% (86/449) of Iclusig-treated patients, of which 7% (33/449) were Grade 3 or greater. Arrhythmia of ventricular origin was reported in 3% (3/86) of all arrhythmias, with one case being Grade 3 or greater.
Symptomatic bradyarrhythmias that led to pacemaker implantation occurred in 1% (3/449) of Iclusig-treated patients.
Atrial fibrillation was the most common arrhythmia and occurred in 7% (31/449) of patients, approximately half of which were Grade 3 or 4. Other Grade 3 or 4 arrhythmia events included syncope (9 patients; 2.0%), tachycardia and bradycardia (2 patients each 0.4%), and electrocardiogram QT prolonged, atrial flutter, supraventricular tachycardia, ventricular tachycardia, atrial tachycardia, atrioventricular block complete, cardio-respiratory arrest, loss of consciousness, and sinus node dysfunction (1 patient each 0.2%). For 27 patients, the event led to hospitalization.
In patients with signs and symptoms suggestive of slow heart rate (fainting, dizziness) or rapid heart rate (chest pain, palpitations or dizziness), interrupt Iclusig and evaluate.
5.13 Myelosuppression
Myelosuppression was reported as an adverse reaction in 59% (266/449) of patients, and severe (Grade 3 or 4) myelosuppression occurred in 50% (226/449) of patients treated with Iclusig. With 48 months of follow-up, the incidence of these events was greater in patients with AP-CML, BP-CML, and Ph+ ALL than in patients with CP-CML.
Severe myelosuppression (Grade 3 or 4) was observed early in treatment, with a median onset time of 1 month (range <1 to 40 months). Obtain complete blood counts every 2 weeks for the first 3 months and then monthly or as clinically indicated, and adjust the dose as recommended [see Dosage and Administration (2.2)].
5.14 Tumor Lysis Syndrome
Two patients (<1%) treated with Iclusig developed serious tumor lysis syndrome. One case occurred in a patient with advanced AP-CML and one case occurred in a patient with BP-CML. Hyperuricemia occurred in 7% (31/449) of patients. Due to the potential for tumor lysis syndrome in patients with advanced disease (AP-CML, BP-CML, or Ph+ ALL), ensure adequate hydration and treat high uric acid levels prior to initiating therapy with Iclusig.
5.15 Reversible Posterior Leukoencephalopathy Syndrome (RPLS)
Postmarketing cases of reversible posterior leukoencephalopathy syndrome (RPLS – also known as Posterior Reversible Encephalopathy Syndrome – PRES) have been reported in Iclusig-treated patients. RPLS is a neurological disorder that can present with signs and symptoms such as seizure, headache, decreased alertness, altered mental functioning, vision loss, and other visual and neurological disturbances. Hypertension is often present and diagnosis is made with supportive findings on magnetic resonance imaging (MRI) of the brain. If RPLS is diagnosed, interrupt Iclusig treatment and resume treatment only once the event is resolved and if the benefit of continued treatment outweighs the risk of RPLS.
5.16 Impaired Wound Healing and Gastrointestinal Perforation
Impaired wound healing occurred in patients receiving Iclusig [see Adverse Reactions (6.2)]. Withhold Iclusig for at least 1 week prior to elective surgery. Do not administer for at least 2 weeks following major surgery and until adequate wound healing. The safety of resumption of Iclusig after resolution of wound healing complications has not been established.
Gastrointestinal perforation or fistula occurred in patients receiving Iclusig [see Adverse Reactions (6.2)]. Permanently discontinue in patients with gastrointestinal perforation.
5.17 Embryo-Fetal Toxicity
Based on its mechanism of action and findings from animal studies, Iclusig can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, oral administration of ponatinib to pregnant rats during organogenesis caused adverse developmental effects at exposures lower than human exposures at the recommended human dose. Advise pregnant women of the potential risk to the fetus. Advise females of reproductive potential to use effective contraception during treatment with Iclusig and for 3 weeks after the last dose [see Use in Specific Populations (8.1 ,8.3) and Clinical Pharmacology (12.1)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Arterial Occlusion [see Warnings and Precautions (5.1)]
- Venous Thromboembolism [see Warnings and Precautions (5.2)]
- Heart Failure [see Dosage and Administration (2.3) and Warnings and Precautions (5.3)]
- Hepatotoxicity [see Dosage and Administration (2.3) and Warnings and Precautions (5.4)]
- Hypertension [see Warnings and Precautions (5.5)]
- Pancreatitis [see Dosage and Administration (2.3) and Warnings and Precautions (5.6)]
- Increased Toxicity in Newly Diagnosed Chronic Phase CML [see Warnings and Precautions (5.7)]
- Neuropathy [see Warnings and Precautions (5.8)]
- Ocular Toxicity [see Warnings and Precautions (5.9)]
- Hemorrhage [see Warnings and Precautions (5.10)]
- Fluid Retention [see Warnings and Precautions (5.11)]
- Cardiac Arrhythmias [see Warnings and Precautions (5.12)]
- Myelosuppression [see Dosage and Administration (2.2) and Warnings and Precautions (5.13)]
- Tumor Lysis Syndrome [see Warnings and Precautions (5.14)]
- Reversible Posterior Leukoencephalopathy Syndrome [see Warnings and Precautions (5.15)]
- Impaired Wound Healing and Gastrointestinal Perforation [see Warnings and Precautions (5.16)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Previously Treated CML or Ph+ ALL
The adverse reactions described in this section were identified in a single-arm, open-label, international, multicenter trial in 449 patients with CML or Ph+ ALL whose disease was considered to be resistant or intolerant to prior tyrosine kinase inhibitor (TKI) therapy including those with the BCR-ABL T315I mutation.
All patients received a starting dose of 45 mg Iclusig once daily. Interruptions and dose adjustments to 30 mg once daily or 15 mg once daily were allowed for the management of treatment toxicity. Additionally, after approximately 2 years of follow-up, patients who were still taking a 45 mg daily dose were recommended to undergo a dose reduction, in response to the continued occurrence of arterial occlusive events and venous thromboembolic events in the clinical trial.
At the time of analysis (48 months of follow-up), 133 patients (30%) were ongoing (110 CP-CML; 20 AP-CML; 3 BP-CML; 0 Ph+ ALL), and the median duration of treatment with Iclusig was 32.2 months in patients with CP-CML, 19.4 months in patients with AP-CML, 2.9 months in patients with BP-CML, and 2.7 months in patients with Ph+ ALL. The median dose intensity in patients with CP-CML was 29 mg /day or 64% of the 45 mg starting dose; median dose intensity was greater in patients with advanced disease patients. Seventy-one percent (318/449) of patients experienced a dose interruption of more than 3 days and 68% (304/449) experienced a dose reduction.
The most common adverse reactions (≥5%) that led to dose modifications (interruption or dose reduction) include thrombocytopenia (31%), neutropenia (14%), lipase increased (13%), arterial occlusive events (13%), abdominal pain (12%), rash (9%), anemia (6%), pancreatitis (6%), ALT increased (5%) and hypertension (5%).
At the time of the analysis, 69% of the ongoing patients (92/133 patients) were reported to be receiving 15 mg; with 26% (35/133) and 5% (6/133) of Iclusig-treated patients receiving 30 mg and 45 mg, respectively.
Adverse reactions reported in more than 10% of all patients treated with Iclusig in this trial are presented in Table 5. Overall, the most common non-hematologic adverse reactions (≥20%) were abdominal pain, rash, constipation, headache, dry skin, arterial occlusion, fatigue, hypertension, pyrexia, arthralgia, nausea, diarrhea, lipase increased, vomiting, myalgia and pain in extremity.
The rates of treatment-emergent adverse reactions resulting in discontinuation were 19% in CP-CML, 12% in AP-CML, 15% in BP-CML, and 9% in Ph+ ALL. The most common adverse reactions that led to treatment discontinuation was thrombocytopenia (4%).
Table 5: Adverse Reactions in >10% of Patients in the Phase 2 Trial (N=449) CP-CML
(N=270)AP-CML
(N=85)BP-CML
(N=62)Ph+ ALL
(N=32)Body System Any Grade
(%)Grade 3/4
(%)Any Grade
(%)Grade 3/4
(%)Any Grade
(%)Grade 3/4
(%)Any Grade
(%)Grade 3/4
(%)Adverse drug reactions, reported using MedDRA and graded using NCI-CTC-AE v 4.0 (NCI Common Terminology Criteria for Adverse Events) for assessment of toxicity. Treatment-emergent, all causality events - * Derived from blood pressure (BP) measurement recorded monthly while on trial
- † Cardiovascular, cerebrovascular, and peripheral vascular ischemia
- ‡ Includes cardiac failure, cardiac failure congestive, cardiogenic shock, cardiopulmonary failure, ejection fraction decreased, pulmonary edema, right ventricular failure
- § Includes abdominal pain, abdominal pain upper, abdominal pain lower, abdominal discomfort
- ¶ Includes aphthous stomatitis, lip blister, mouth ulceration, oral mucosal eruption, oral pain, oropharyngeal pain, pharyngeal ulceration, stomatitis, tongue ulceration
- # Includes gastric hemorrhage, gastric ulcer hemorrhage, hemorrhagic gastritis, gastrointestinal hemorrhage, hematemesis, hematochezia, hemorrhoidal hemorrhage, intra-abdominal hemorrhage, melena, rectal hemorrhage, and upper gastrointestinal hemorrhage
- Þ Includes burning sensation, skin burning sensation, hyperesthesia, hypoesthesia, neuralgia, neuropathy peripheral, paresthesia, peripheral sensorimotor neuropathy, peripheral motor neuropathy, peripheral sensory neuropathy, polyneuropathy, dysgeusia, muscular weakness, gait disturbance, nerve compression, areflexia, hypotonia, restless legs syndrome
Cardiac or Vascular Disorders Hypertension* 69 42 74 42 60 23 53 28 Arterial ischemia† 42 17 28 14 11 8 22 6 Cardiac failure‡ 8 5 7 5 15 8 6 3 Gastrointestinal Disorders Abdominal pain§ 48 10 42 9 35 8 34 6 Constipation 41 3 27 2 27 0 53 3 Nausea 28 1 31 0 34 2 22 0 Diarrhea 20 1 29 2 24 3 13 3 Vomiting 18 2 26 0 27 2 25 0 Oral mucositis¶ 14 1 19 1 23 0 9 3 GI hemorrhage# 1 <1 8 1 6 3 9 6 Blood and Lymphatic System Disorders Febrile neutropenia 1 1 5 5 13 13 25 25 Infections and Infestations Sepsis 2 1 4 4 3 0 13 13 Pneumonia 6 5 13 9 16 11 9 3 Urinary tract infection 11 2 14 2 2 2 9 0 Upper respiratory tract infection 14 1 13 0 13 2 3 0 Nasopharyngitis 12 0 18 0 3 0 3 0 Cellulitis 3 2 6 2 11 3 0 0 Nervous System Disorders Headache 43 3 29 0 31 3 25 0 Peripheral neuropathyÞ 24 3 14 1 11 0 16 0 Dizziness 16 0 9 0 5 0 3 0 Respiratory, Thoracic, and Mediastinal Disorders Pleural effusion 5 2 12 2 13 0 19 3 Cough 16 0 22 0 19 0 6 0 Dyspnea 17 3 20 4 19 5 6 0 Skin and Subcutaneous Tissue Disorders Rash and related conditions 63 4 59 7 39 5 28 3 Dry skin 42 3 32 1 26 2 25 0 Pruritus 13 <1 8 0 5 2 0 0 Erythema 10 1 8 0 8 0 6 0 Alopecia 7 0 11 0 8 0 6 0 Musculoskeletal and Connective Tissue Disorders Arthralgia 32 3 33 2 19 0 13 0 Myalgia 24 1 20 0 18 0 6 0 Pain in extremity 23 3 19 0 13 0 13 0 Back pain 21 1 14 2 19 2 13 0 Muscle spasms 14 0 6 0 5 0 13 0 Bone pain 14 <1 13 1 11 3 9 3 Musculoskeletal pain 11 2 7 0 8 0 6 3 General Disorders and Administration Site Conditions Fatigue or asthenia 47 4 49 8 40 6 34 3 Pyrexia 26 1 40 7 36 3 25 0 Edema, peripheral 16 <1 18 0 15 0 25 0 Pain 10 <1 13 0 16 3 6 0 Chills 8 0 11 0 13 2 9 0 Metabolism and Nutrition Disorders Decreased appetite 13 <1 14 1 8 0 31 0 Investigations Weight decreased 10 <1 9 0 5 0 13 0 Psychiatric Disorders Insomnia 11 0 13 0 11 0 13 0 Table 6: Serious Adverse Reactions Occurring in >2% of Patients from the Phase 2 Trial (N=449) System Organ Class N (%) - * Includes pericardial effusion, pleural effusion, and ascites
Cardiovascular Disorders Arterial occlusion 99 (22%) Cardiac vascular 53 (12%) Cerebrovascular 31 (7%) Peripheral vascular 34 (8%) Venous thromboembolism 22 (5%) Hemorrhage 28 (6%) CNS hemorrhage 6 (1%) Gastrointestinal hemorrhage 11 (2%) Heart failure 28(6%) Effusions* 15 (3%) Atrial fibrillation 18 (4%) Hypertension 12 (3%) Gastrointestinal Disorders Pancreatitis 26(6%) Abdominal pain 20 (5%) Blood and Lymphatic System Disorders Febrile neutropenia 13 (3%) Anemia 16(3%) Thrombocytopenia 14 (3%) Infections Pneumonia 32 (7%) Sepsis 10 (2%) General Pyrexia 20 (5%) Laboratory Abnormalities
Myelosuppression was commonly reported in all patient populations. The frequency of Grade 3 or 4 thrombocytopenia, neutropenia, and anemia was higher in patients with AP-CML, BP-CML, and Ph+ ALL than in patients with CP-CML (see Table 7).
Table 7: Clinically Relevant Grade 3/4* Hematologic Laboratory Abnormalities in Patients from the Phase 2 Trial (N=449) Laboratory Test CP-CML
(N=270)
(%)AP-CML
(N=85)
(%)BP-CML
(N=62)
(%)Ph+ ALL
(N=32)
(%)ANC = absolute neutrophil count, Hgb = hemoglobin, WBC = white blood cell count - * Reported using NCI-CTC-AE v 4.0
Hematology Thrombocytopenia
(platelet count decreased)35 49 45 47 Neutropenia
(ANC decreased)23 52 48 59 Leukopenia
(WBC decreased)12 36 48 63 Anemia
(Hgb decreased)8 31 52 34 Lymphopenia 10 25 32 19 Table 8: Clinically Relevant Non-hematologic Laboratory Abnormalities Laboratory Test Safety Population
N=449Any Grade*
(%)CTCAE Grade 3/4
(%)ALT = alanine aminotransferase, AST=aspartate aminotransferase - * Graded using NCI-CTC-AE v4.0
Liver Function Tests ALT increased 41 6 AST increased 35 4 Alkaline phosphatase increased 40 2 Albumin decreased 27 <1 Bilirubin increased 13 <1 Pancreatic Enzymes Lipase increased 38 13 Amylase increased 18 3 Chemistry Glucose increased 54 7 Phosphorus decreased 33 10 Calcium decreased 30 <1 Sodium decreased 27 5 Glucose decreased 13 0 Potassium decreased 18 2 Potassium increased 19 2 Sodium increased 10 <1 Bicarbonate decreased 19 <1 Creatinine increased 21 <1 Calcium increased 12 0 Triglycerides increased 3 <1 6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of Iclusig. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
Nervous System Disorders: Reversible posterior leukoencephalopathy syndrome (RPLS) – also known as Posterior Reversible Encephalopathy Syndrome (PRES)
Metabolism and Nutrition Disorders: Dehydration
Skin and Subcutaneous Tissue Disorders: Severe cutaneous reaction (e.g., Erythema multiforme, Stevens-Johnson syndrome), impaired wound healing
Blood and Lymphatic System Disorders: Thrombotic microangiopathy
Gastrointestinal Disorders: Gastrointestinal perforation, fistula
-
7 DRUG INTERACTIONS
7.1 Drugs that are Strong Inhibitors of CYP3A Enzymes
Based on in vitro studies, ponatinib is a substrate of CYP3A and to a lesser extent CYP2C8 and CYP2D6. In a drug interaction study in healthy volunteers, coadministration of Iclusig with ketoconazole increased plasma ponatinib AUC0-inf and Cmax by 78% and 47%, respectively [see Clinical Pharmacology (12.3)]. When administering Iclusig with strong CYP3A inhibitors (e.g., boceprevir, clarithromycin, conivaptan, grapefruit juice, indinavir, itraconazole, ketoconazole, lopinavir/ritonavir, nefazodone, nelfinavir, posaconazole, ritonavir, saquinavir, telaprevir, telithromycin, voriconazole), the recommended starting dose should be reduced [see Dosage and Administration (2.4)]. Patients taking concomitant strong CYP3A inhibitors may be at increased risk for adverse reactions [see Clinical Pharmacology (12.3)].
7.2 Drugs that are Strong Inducers of CYP3A Enzymes
Coadministration of strong CYP3A inducers (e.g., carbamazepine, phenytoin, rifampin, and St. John's Wort) with Iclusig should be avoided unless the benefit outweighs the risk of decreased ponatinib exposure. Monitor patients for reduced efficacy. Selection of concomitant medication with no or minimal CYP3A induction potential is recommended. In a drug interaction study in healthy volunteers, coadministration of Iclusig following multiple doses of rifampin resulted in decreased ponatinib AUC0-inf and Cmax values by 62% and 42%, respectively [see Clinical Pharmacology (12.3)].
7.3 Drugs that Elevate Gastric pH
Iclusig may be coadministered with gastric pH-elevating medications. In a drug interaction study in healthy volunteers, coadministration of Iclusig following multiple doses of lansoprazole resulted in a minimal (6%) decrease in ponatinib exposure [see Clinical Pharmacology (12.3)].
7.4 Drugs that are Substrates of the P-gp or ABCG2 Transporter Systems
Ponatinib inhibits the P-glycoprotein (P-gp), ATP-binding cassette G2 (ABCG2) [also known as BCRP], and bile salt export pump (BSEP) transporter systems in vitro [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on its mechanism of action and findings in animals, Iclusig can cause fetal harm when administered to a pregnant woman [see Data]. There are no available data on Iclusig use in pregnant women. In animal reproduction studies, oral administration of ponatinib to pregnant rats during organogenesis caused adverse developmental effects at doses lower than human exposures at the recommended human dose [see Data]. Advise pregnant women of the potential risk to a fetus.
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. The background risk in the U.S. general population of major birth defects is 2 to 4% and of miscarriage is 15 to 20% of clinically recognized pregnancies.
Data
Animal Data
Ponatinib was studied for effects on embryo-fetal development in pregnant rats given oral doses of 0.3 mg/kg/day, 1 mg/kg/day, and 3 mg/kg/day during organogenesis (25 rats per group). At the maternally toxic dose of 3 mg/kg/day (equivalent to the AUC in patients receiving the recommended dose of 45 mg/day), ponatinib caused embryo-fetal toxicity as shown by increased resorptions, reduced body weight, external alterations, multiple soft tissue and skeletal alterations, and reduced ossification. Embryo-fetal toxicities also were observed at 1 mg/kg/day (approximately 24% the AUC in patients receiving the recommended dose) and involved multiple fetal soft tissue and skeletal alterations, including reduced ossification.
8.2 Lactation
Risk Summary
There is no data on the presence of ponatinib in human milk, the effects on the breastfed infant or on milk production.
Because of the potential for serious adverse reactions in breastfed infants from ponatinib including arterial occlusion, venous thromboembolism, heart failure, and hepatotoxicity, advise women not to breastfeed during treatment with Iclusig and for 6 days following the last dose.
8.3 Females and Males of Reproductive Potential
Iclusig can cause fetal harm when administered to pregnant women [see Use in Specific Populations (8.1) and Clinical Pharmacology (12.1)].
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating Iclusig treatment.
Infertility
Based on animal data, ponatinib may impair fertility in females of reproductive potential. It is not known whether these effects on fertility are reversible [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness have not been established in pediatric patients.
Juvenile Animal Toxicity Data
A juvenile toxicity study in 15 day old rats was conducted with daily oral gavage administration of ponatinib at 0.75 mg/kg/day, 1.5 mg/kg/day, or 3 mg/kg/day for 21 days. There were no adverse effects of ponatinib on juvenile rat developmental parameters (vaginal opening, preputial separation or bone measurements) observed in this study. Once daily oral administration of 3 mg/kg/day ponatinib to juvenile rats beginning on Day 15 postpartum (pp) resulted in mortality related to inflammatory effects after 6 to 7 days following initiation of treatment. The dose of 3 mg/kg/day is approximately 0.32 times the clinical dose on a mg/m2 basis for a child.
8.5 Geriatric Use
One hundred and fifty-five of 449 patients (35%) in the clinical trial of Iclusig were 65 years of age and over. In patients with CP-CML, patients of age ≥65 years had a lower major cytogenetic response rate (40%) as compared with patients <65 years of age (65%). In patients with AP-CML, BP-CML, and Ph+ ALL, patients of age ≥65 years had a similar hematologic response rate (45%) as compared with patients <65 years of age (44%). Forty percent of patients ≥65 years had arterial occlusion events. Patients of age ≥65 years are more likely to experience adverse reactions including vascular occlusion, decreased platelet count, peripheral edema, increased lipase, dyspnea, asthenia, muscle spasms, and decreased appetite. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
Administer Iclusig at a dose of 30 mg once daily in patients with hepatic impairment (Child-Pugh A, B, or C) [see Dosage and Administration (2.5) and Clinical Pharmacology (12.3)].
In a single-dose (30 mg) pharmacokinetic (PK) study; compared to subjects with normal liver function, no major differences in ponatinib PK were observed in subjects with hepatic impairment (Child-Pugh A, B, or C). However, there was an increased overall incidence of adverse reactions (e.g., gastrointestinal disorders, including a case of severe pancreatitis) in the subjects with hepatic impairment following the single 30 mg dose compared to subjects with normal liver function. The safety of multiple ponatinib doses, or doses higher than 30 mg have not been studied in patients with hepatic impairment.
-
10 OVERDOSAGE
Overdoses with Iclusig were reported in clinical trials. One patient was accidentally administered the entire contents of a bottle of study medication via nasogastric tube. The investigator estimated that the patient received 540 mg of Iclusig. Two hours after the overdose, the patient had an uncorrected QT interval of 520 ms. Subsequent ECGs showed normal sinus rhythm with uncorrected QT intervals of 480 ms and 400 ms. The patient died 9 days after the overdose from pneumonia and sepsis. Another patient accidentally self-administered 165 mg on Cycle 1 Day 2. The patient experienced fatigue and non-cardiac chest pain on Day 3. Multiple doses of 90 mg per day for 12 days in a patient resulted in pneumonia, systemic inflammatory response, atrial fibrillation, and a moderate pericardial effusion.
In the event of an overdose of Iclusig, stop Iclusig, observe the patient and provide appropriate supportive treatment.
-
11 DESCRIPTION
Iclusig (ponatinib) is a kinase inhibitor. The chemical name for ponatinib hydrochloride is 3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N-{4-[(4-methylpiperazin-1-yl)methyl]-3-(trifluoromethyl)phenyl}benzamide hydrochloride. The molecular formula is C29H28ClF3N6O which corresponds to a formula weight of 569.02 g/mol. Its structure is shown below:
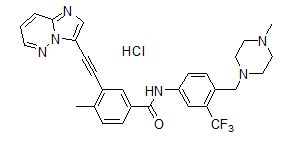
Ponatinib HCl is an off-white to yellow powder with pKa of 2.77 and 7.8. The solubility of ponatinib in pH 1.7, 2.7, and 7.5 buffers is 7790 mcg/mL, 3.44 mcg/mL, and 0.16 mcg/mL, respectively, indicating a decrease in solubility with increasing pH. Iclusig tablets are available as white, round, film-coated tablets for oral administration. Each tablet contains ponatinib hydrochloride equivalent to 15 mg, 30 mg or 45 mg ponatinib with the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, sodium starch glycolate (type B), colloidal silicon dioxide, magnesium stearate and a tablet coating. The tablet coating consists of talc, polyethylene glycol, polyvinyl alcohol, and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Ponatinib is a kinase inhibitor. Ponatinib inhibited the in vitro tyrosine kinase activity of ABL and T315I mutant ABL with IC50 concentrations of 0.4 nM and 2.0 nM, respectively. Ponatinib inhibited the in vitro activity of additional kinases with IC50 concentrations between 0.1 nM and 20 nM, including members of the VEGFR, PDGFR, FGFR, EPH receptors and SRC families of kinases, and KIT, RET, TIE2, and FLT3. Ponatinib inhibited the in vitro viability of cells expressing native or mutant BCR-ABL, including T315I. In mice, treatment with ponatinib reduced the size of tumors expressing native or T315I mutant BCR-ABL when compared to controls.
12.2 Pharmacodynamics
In a cell-based assay, ponatinib concentrations of 20 nM (10.65 ng/mL) were sufficient to suppress most BCR-ABL mutant clones. However, ponatinib concentrations of 40 nM (21.3 ng/mL) were required to suppress T315I mutants. The median and range of steady state Cmax and trough (Cmin) concentrations of ponatinib following 29 days of once daily dosing of 15 mg, 30 mg and 45 mg are listed in Table 10.
Table 10: Median, Maximum, and Minimum Ponatinib Exposure at Steady State by Dose Group: PK Evaluable Population Dose Median Cmax (Range)
(nM)Median Cmin (Range)
(nM)15 mg QD (n=8) 49 (23 to 105) 28 (11 to 68) 30 mg QD (n=9) 125 (67 to 178) 54 (41 to 89) 45 mg QD (n=21) 161 (64 to 336) 67 (22 to 137) Concentrations of ponatinib shown in cell-based assays to suppress unmutated BCR-ABL and most mutant BCR-ABL clones may be achieved at once daily dosing of 15 mg or 30 mg.
The dose intensity-safety relationship indicated that there are significant increases in Grade ≥3 adverse events (hypertension, thrombocytopenia, pancreatitis, neutropenia, rash, ALT increase, AST increase, lipase increase, myelosuppression) over the dose range of 15 mg to 45 mg once daily.
In vitro, there was no significant inhibition of platelet aggregation with ponatinib at concentrations seen clinically and up to 0.7 mcg/mL (1.23 μM).
Cardiac Electrophysiology
A QT assessment was performed in 39 patients with cancer who received 30 mg, 45 mg, or 60 mg Iclusig once daily. No large changes in the mean QTc interval (i.e., >20 msec) from baseline were detected in the study. However, a small increase in the mean QTc interval (i.e., <10 msec) cannot be excluded because of study design limitations. In a Phase 3 trial comparing ponatinib with imatinib, the mean change from baseline to worst QTcF value in ponatinib-treated patients (n=124) was <10 msec.
12.3 Pharmacokinetics
The geometric mean (CV%) Cmax and AUC(0-τ) of Iclusig 45 mg daily at presumed steady state in patients with advanced hematologic malignancies were 73 ng/mL (74%) and 1253 ng∙hr/mL (73%), respectively. Ponatinib administered as an investigational capsule formulation to patients with cancer exhibited approximately dose proportional increases in both Cmax and AUC over the dose range of 15 mg to 60 mg. A dose intensity safety analysis showed a significant increase in Grade 3 or higher adverse reactions (i.e., thrombocytopenia, neutropenia, rash, ALT elevation, AST elevation, pancreatitis, and lipase elevation) with an increase in dose intensity.
Absorption
The absolute bioavailability of ponatinib is unknown. Peak concentrations of ponatinib are observed within 6 hours after Iclusig oral administration. Following ingestion of either a high fat or low fat meal by 22 healthy volunteers, plasma ponatinib exposures (AUC and Cmax) were not different when compared to fasting conditions.
Distribution
Ponatinib is greater than 99% bound to plasma proteins in vitro. There was no plasma protein binding displacement of ponatinib (145 nM) in vitro by other highly protein bound medications (ibuprofen, nifedipine, propranolol, salicylic acid, and warfarin). The geometric mean (CV%) apparent steady state volume of distribution is 1223 liters (102%) following oral administration of Iclusig 45 mg once daily for 28 days in patients with cancer. Ponatinib is a weak substrate for both P-gp and ABCG2 in vitro. Ponatinib is not a substrate for organic anion transporting polypeptides (OATP1B1, OATP1B3) and organic cation transporter 1 (OCT1) in vitro.
Metabolism
At least 64% of a ponatinib dose undergoes Phase I and Phase II metabolism. CYP3A4 and to a lesser extent CYP2C8, CYP2D6 and CYP3A5 are involved in the Phase I metabolism of ponatinib in vitro. Ponatinib is also metabolized by esterases and/or amidases.
Elimination
The geometric mean (range) terminal elimination half-life of ponatinib was approximately 24 (12 to 66) hours following Iclusig 45 mg oral administration once daily for 28 days in patients with cancer. Exposure increased by approximately 90% (median) [range: 20% to 440%] between the first dose and presumed steady state. Ponatinib is mainly eliminated via feces. Following a single oral dose of [14C]-labeled ponatinib, approximately 87% of the radioactive dose is recovered in the feces and approximately 5% in the urine.
Drug Interaction Studies
Coadministration of Ponatinib and CYP3A Inhibitors
Coadministration of a single 15 mg oral dose of ponatinib in the presence of ketoconazole (400 mg daily), a strong CYP3A inhibitor, to 22 healthy volunteers, increased the AUC0-inf and Cmax of ponatinib by 78% and 47%, respectively, when compared to administration of ponatinib alone [see Drug Interactions (7.1)].
Coadministration of Ponatinib and CYP3A Inducers
Coadministration of a single 45 mg dose of ponatinib in the presence of rifampin (600 mg daily), a strong CYP3A inducer, to 19 healthy volunteers, decreased the AUC0-inf and Cmax of ponatinib by 62% and 42%, respectively, when compared to administration of ponatinib alone [see Drug Interactions (7.2)].
Coadministration with Other CYP Substrates
In vitro studies indicate that ponatinib does not inhibit the metabolism of substrates for CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP3A, or CYP2D6 and does not induce the metabolism of substrates for CYP1A2, CYP2B6, or CYP3A.
Coadministration with Substrates of Transporters
In vitro, ponatinib is an inhibitor of P-gp and ABCG2, and BSEP [see Drug Interactions (7.4)].
In vitro, ponatinib did not inhibit the human organic anion transporting polypeptides OATP1B1 or OATP1B3, or the organic cation transporters OCT1, OCT2, OAT1, and OAT3.
Coadministration of Ponatinib and Gastric pH-Elevating Medications
The aqueous solubility of ponatinib is pH-dependent, with higher pH resulting in lower solubility [see Description (11)]. Coadministration of a single 45 mg dose of ponatinib in the presence of lansoprazole (60 mg daily), a proton pump inhibitor, to 18 healthy volunteers decreased the AUC0-inf and Cmax of ponatinib by 6% and 25%, respectively, when compared to administration of ponatinib alone [see Drug Interactions (7.3)].
Pharmacokinetics in Specific Populations
Hepatic Impairment
A single 30 mg oral dose of ponatinib was administered to subjects with normal liver function (N=8) and to subjects with mild [Child-Pugh A (N=6)], moderate [Child-Pugh B (N=6)], and severe [Child-Pugh C (N=4)] hepatic impairment. Compared to subjects with normal liver function, there was no trend of increased ponatinib exposure in subjects with hepatic impairment. There was an increased incidence of adverse reactions in patients with hepatic impairment compared to subjects with normal liver function [see Dosage and Administration (2.5) and Use in Specific Populations (8.6)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year carcinogenicity study, male and female rats were administered daily oral doses of ponatinib of 0.05 mg/kg/day, 0.1 mg/kg/day, 0.2 mg/kg/day and 0.2 mg/kg/day, 0.4 mg/kg/day, and 0.8 mg/kg/day, respectively. Exposures in animals at the highest dose tested were 0.3- to 0.8-fold the human exposure (based on AUC) at doses of 15 mg and 45 mg daily. Ponatinib induced a statistically significant increase in malignant squamous neoplasms of the clitoral gland in females at 0.8 mg/kg/day.
Ponatinib was not mutagenic in a bacterial mutagenesis (Ames) assay, was not clastogenic in a chromosome aberration assay in human lymphocytes, nor was it clastogenic in an in vivo mouse micronucleus assay at oral doses up to 2000 mg/kg.
Ponatinib may impair female fertility. In a fertility study in male and female rats, female fertility parameters were reduced at 1.5 mg/kg/day with exposure equivalent to 0.43 times and 1.23 times, of human daily steady state AUC at the recommended dose of 45 mg/day (AUC = 1296 h∙ng/mL) and 15 mg/day (451.8 h∙ng/mL), respectively. Evidence of pre- and postimplantation loss of embryos was observed in female rats. Although there were no effects on male fertility parameters in the rat fertility study, repeat dose toxicology studies in monkeys showed degeneration of epithelium of the testes in monkeys at exposures approximately 3.3 times the plasma drug exposure (AUC) in patients receiving the recommended dose of 45 mg/day.
-
14 CLINICAL STUDIES
The safety and efficacy of Iclusig in patients with CML and Ph+ ALL whose disease was considered to be resistant or intolerant to prior tyrosine kinase inhibitor (TKI) therapy were evaluated in a single-arm, open-label, international, multicenter trial. Efficacy results described below should be interpreted within the context of updated safety information [see Boxed Warning, Dosage and Administration (2.1) and Warnings and Precautions (5.1, 5.2)].
All patients were administered a starting dose of 45 mg of Iclusig once daily. Patients were assigned to one of six cohorts based on disease phase (chronic phase CML [CP-CML]; accelerated phase CML [AP-CML]; or blast phase CML/Philadelphia-positive acute lymphoblastic leukemia [BP-CML/Ph+ ALL]), resistance or intolerance (R/I) to prior TKI therapy, and the presence of the T315I mutation.
Resistance in CP-CML while on prior TKI therapy, was defined as failure to achieve either a complete hematologic response (by 3 months), a minor cytogenetic response (by 6 months), or a major cytogenetic response (by 12 months). Patients with CP-CML who experienced a loss of response or development of a kinase domain mutation in the absence of a complete cytogenetic response or progression to AP-CML or BP-CML at any time on prior TKI therapy were also considered resistant. Resistance in AP-CML, BP-CML, and Ph+ ALL was defined as failure to achieve either a major hematologic response (by 3 months in AP-CML, and by 1 month in BP-CML and Ph+ ALL), loss of major hematologic response (at any time), or development of a kinase domain mutation in the absence of a complete major hematologic response while on prior TKI therapy.
Intolerance was defined as the discontinuation of prior TKI therapy due to toxicities despite optimal management in the absence of a complete cytogenetic response in patients with CP-CML or major hematologic response for patients with AP-CML, BP-CML, or Ph+ ALL.
The primary efficacy endpoint in CP-CML was major cytogenetic response (MCyR), which included complete and partial cytogenetic responses (CCyR and PCyR). The primary efficacy endpoint in AP-CML, BP-CML, and Ph+ ALL was major hematologic response (MaHR), defined as either a complete hematologic response (CHR) or no evidence of leukemia (NEL).
The trial enrolled 449 patients, of which 444 were eligible for efficacy analysis: 267 patients with CP-CML (R/I Cohort: n=203, T315I: n=64), 83 patients with AP-CML, 62 patients with BP-CML, and 32 patients with Ph+ ALL. Five patients were not eligible for efficacy analysis due to lack of confirmation of T315I mutation status, and these patients had not received prior dasatinib or nilotinib.
At the time of analysis, the median duration of follow-up for the trial (all cohorts) was 37.3 months (minimum of 48 months of follow-up for all ongoing patients). Baseline demographic characteristics are described in Table 11.
Table 11: Demographic and Disease Characteristics Patient Characteristics at Entry Efficacy Population
N=444- * Of the patients with one or more BCR-ABL kinase domain mutations detected at entry, 37 unique mutations were detected.
Age Median, years (range) 59 (18 to 94) Gender, n (%) Male 236 (53%) Race, n (%) Asian 57 (13%) Black or African American 25 (6%) White 349 (79%) Other 13 (3%) ECOG Performance Status, n (%) ECOG=0 or 1 409 (92%) Disease History Median time from diagnosis to first dose, years (range) 6.1 (0.3 to 28.5) Resistant to Prior TKI Therapy, n (%) 374 (88%) Presence of one or more BCR-ABL kinase domain mutations* 244 (55%) Prior TKI therapy – number of prior approved TKIs, n (%) 1 29 (7%) 2 166 (37%) ≥3 249 (56%) At the time of analysis, there were 133 patients ongoing (110 patients with CP-CML; 20 patients with AP-CML; 3 patients with BP-CML; 0 patients with Ph+ ALL), and the median duration of Iclusig treatment was 32.2 months in patients with CP-CML, 19.4 months in patients with AP-CML, 2.9 months in patients with BP-CML and 2.7 months in patients with Ph+ ALL.
Efficacy results are summarized in Table 12, and Table 13.
Table 12: Efficacy of Iclusig in Patients with Resistant or Intolerant Chronic Phase CML Overall
(N=267)Cohort R/I Cohort
(N=203)T315I Cohort
(N=64)- * Primary endpoint for CP-CML Cohorts was MCyR by 12 months, which combines both complete (no detectable Ph+ cells) and partial (1% to 35% Ph+ cells in at least 20 metaphases) cytogenetic responses.
- † Secondary endpoint for CP-CML Cohorts was MMR (proportion of patients who met the criteria for MMR at least once after the initiation of study treatment) measured in peripheral blood. Defined as a ≤0.1% ratio of BCR-ABL to ABL transcripts on the International Scale (IS) (i.e., ≤0.1% BCR-ABLIS; patients must have the b2a2/b3a2 (p210) transcript), in peripheral blood measured by quantitative reverse transcriptase polymerase chain reaction (qRT PCR).
Cytogenetic Response Major * (MCyR)
%
(95% CI)55%
(49,62)51%
(44,58)70%
(58,81)Complete (CCyR)
%
(95% CI)46%
(40,52)40%
(33,47)66%
(53,77)Major Molecular Response †
%
(95% CI)39%
(33,46)34%
(27,40)58%
(45,70)In patients with CP-CML who achieved MCyR or MMR, the median time to response was 2.8 months (range: 1.6 to 11.3 months) and 5.5 months (range: 1.8 to 47.4 months), respectively. With a minimum follow-up of 48 months, the median durations of MCyR (range: 2.7 to 50.3+ months) and MMR (range: 1.7 to 50.3+ months) had not yet been reached.
Table 13: Efficacy of Iclusig in Patients with Resistant or Intolerant Advanced Disease (includes R/I and T315I cohorts) AP-CML Overall
(N=83)BP-CML Overall
(N=62)Ph+ ALL Overall
(N=32)- * Primary endpoint for patients with AP-CML, BP-CML, and Ph+ ALL was MaHR by 6 months, which combines complete hematologic responses and no evidence of leukemia.
- † CHR: WBC ≤ institutional ULN, ANC ≥1000/mm3, platelets ≥100,000/mm3, no blasts or promyelocytes in peripheral blood, bone marrow blasts ≤5%, <5% myelocytes plus metamyelocytes in peripheral blood, basophils <5% in peripheral blood, no extramedullary involvement (including no hepatomegaly or splenomegaly).
Hematologic Response Major* (MaHR) % 57% 31% 41% (95% CI) (45,68) (20,44) (24,59) Complete† (CHR) % 51% 21% 34% (95% CI) (39, 62) (12,33) (19,53) The median time to MaHR in patients with AP-CML, BP-CML, and Ph+ ALL was 0.7 months (range: 0.4 to 5.8 months), 1.0 month (range 0.4 to 3.7 months), and 0.7 months (range: 0.4 to 5.5 months), respectively. The median duration of MaHR for patients with AP-CML, BP-CML, and Ph+ ALL was 12.9 months (range: 1.2 to 52+ months), 6.0 months (range: 1.8 to 47.4+ months), and 3.2 months (range: 1.8 to 12.8+ months), respectively.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Iclusig tablets are available in the following configurations.
Strength NDC Number Description Presentation 15 mg 63020-535-30 round, white, film-coated tablets with debossed "A5" on one side and plain on the other side 30 tablets in a wide-mouth white high density polyethylene (HDPE) bottle with a desiccant canister and induction sealed child resistant closure. 63020-535-60 60 tablets in a wide-mouth white high density polyethylene (HDPE) bottle with a desiccant canister and induction sealed child resistant closure. 30 mg 63020-533-30 round, white, film-coated tablets with debossed "C7" on one side and plain on the other side 30 tablets in a wide-mouth white high density polyethylene (HDPE) bottle with a desiccant canister and induction sealed child resistant closure. 45 mg 63020-534-30 round, white, film-coated tablets with debossed "AP4" on one side and plain on the other side 30 tablets in a wide-mouth white high density polyethylene (HDPE) bottle with a desiccant canister and induction sealed child resistant closure. -
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Arterial Occlusions and Venous Thromboembolism
Inform patients that serious arterial thromboses (including arterial stenosis sometimes requiring revascularization) and venous thromboembolism events have occurred. Advise patients to immediately contact their healthcare provider with any symptoms suggestive of a blood clot such as chest pain, shortness of breath, weakness on one side of the body, speech problems, leg pain, or leg swelling [see Warnings and Precautions (5.1, 5.2)].
Heart Failure and Cardiac Arrhythmias
Inform patients of the possibility of heart failure, and abnormally slow or fast heart rates. Advise patients to contact their healthcare provider if they experience symptoms such as shortness of breath, chest pain, palpitations, dizziness, or fainting [see Warnings and Precautions (5.3, 5.11)].
Hepatotoxicity
Inform patients of the possibility of developing liver function abnormalities and serious hepatic toxicity. Advise patients to immediately contact their healthcare provider if signs of liver failure occur, including jaundice, anorexia, bleeding or bruising [see Warnings and Precautions (5.4)].
Hypertension
Inform patients of the possibility of new or worsening of existing hypertension. Advise patients to contact their healthcare provider for elevated blood pressure or if symptoms of hypertension occur including confusion, headache, dizziness, chest pain, or shortness of breath [see Warnings and Precautions (5.5)].
Pancreatitis
Inform patients of the possibility of developing pancreatitis that may be accompanied by nausea, vomiting, abdominal pain, or abdominal discomfort, and to promptly report these symptoms [see Warnings and Precautions (5.6)].
Neuropathy
Inform patients of the possibility of developing peripheral or cranial neuropathy while being treated with Iclusig. Advise patients to report symptoms of neuropathy, such as hypoesthesia, hyperesthesia, paresthesia, discomfort, a burning sensation, neuropathic pain, or weakness [see Warnings and Precautions (5.8)].
Ocular Toxicity
Inform patients of the possibility of ocular toxicity while being treated with Iclusig. Advise patients to report symptoms of ocular toxicity, such as blurred vision, dry eye, or eye pain [see Warnings and Precautions (5.9)].
Hemorrhage
Inform patients of the possibility of serious bleeding and to immediately contact their healthcare provider with any signs or symptoms suggestive of hemorrhage such as unusual bleeding or easy bruising [see Warnings and Precautions (5.10)].
Fluid Retention
Inform patients of the possibility of developing fluid retention and to contact their healthcare provider for symptoms such as leg swelling, abdominal swelling, weight gain, or shortness of breath [see Warnings and Precautions (5.11)].
Myelosuppression
Inform patients of the possibility of developing low blood cell counts; inform patients to report immediately should fever develop, particularly in association with any suggestion of infection [see Warnings and Precautions (5.13)].
Reversible Posterior Leukoencephalopathy Syndrome (RPLS – also known as Posterior Reversible Encephalopathy Syndrome – PRES)
Inform patients of the possibility of developing Reversible Posterior Leukoencephalopathy Syndrome while being treated with Iclusig. Advise patients to report symptoms such as seizure, headache, decreased alertness, altered mental functioning, vision loss, and other visual and neurological disturbances [see Warnings and Precautions (5.15)].
Impaired Wound Healing and Gastrointestinal Perforation
Inform patients that impaired wound healing and gastrointestinal fistula or perforation have been reported. Advise patients to inform their healthcare provider of any planned surgical procedure [see Warnings and Precautions (5.16)].
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with Iclusig and for 3 weeks after the last dose. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.17) and Use in Specific Populations (8.1, 8.3)].
Lactation
Advise women not to breastfeed during treatment with Iclusig and for 6 days after the last dose [see Use in Specific Populations (8.2)].
Infertility
Advise females of reproductive potential of the potential for reduced fertility from Iclusig [see Use in Specific Populations (8.3) and Nonclinical Toxicology (13.1)].
Instructions for Taking Iclusig
Advise patients to take Iclusig exactly as prescribed and not to change their dose or to stop taking Iclusig unless they are told to do so by their healthcare provider. Iclusig may be taken with or without food. Iclusig tablets should be swallowed whole. Patients should not crush or dissolve the tablets.
Patients should not take two doses at the same time to make up for a missed dose.
-
SPL UNCLASSIFIED SECTION
Manufactured for:
Takeda Pharmaceutical Company Limited
40 Landsdowne Street Cambridge, MA 02139-4234Iclusig® is a registered Trademark of ARIAD Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited
©2012 – 2020 ARIAD Pharmaceuticals Inc. All rights reserved.
ICL348 R3
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: January 2020 MEDICATION GUIDE
ICLUSIG® (eye-CLUE-sig)
(ponatinib)
tabletsWhat is the most important information I should know about Iclusig?
Iclusig can cause serious side effects, including:
Blood clots or blockage in your blood vessels (arteries and veins). Blood clots or blockage in your blood vessels may lead to heart attack, stroke, or death. A blood clot or blockage in your blood vessels can prevent proper blood flow to your heart, brain, bowels (intestines), legs, eyes, and other parts of your body. You may need emergency surgery or treatment in a hospital. Get medical help right away if you get any of the following symptoms:- chest pain or pressure
- pain in your arms, legs, back, neck or jaw
- shortness of breath
- numbness or weakness on one side of your body
- leg swelling
- trouble talking
- headache
- dizziness
- severe stomach area pain
- decreased vision or loss of vision
Blood clots or blockage in your blood vessels can happen in people with or without risk factors for heart and blood vessel disease, including people 50 years of age or younger. The most common risk factors for these problems are a history of high blood pressure (hypertension), high levels of fat in the blood (hyperlipidemia), and heart disease. Blood clots or blockages in your blood vessels happen more often in people as they get older, and in people with a past history of decreased blood flow, high blood pressure, diabetes, or high levels of fats in the blood.
Heart problems. Iclusig can cause heart problems, including heart failure which can be serious and may lead to death. Heart failure means your heart does not pump blood well enough. Iclusig can also cause irregular slow or fast heartbeats and heart attack. Your healthcare provider will check you for heart problems during your treatment with Iclusig. Get medical help right away if you get any of the following symptoms: shortness of breath, chest pain, fast or irregular heartbeats, dizziness, or feel faint.
Liver problems. Iclusig can cause liver problems, including liver failure, which can be severe and may lead to death. Your healthcare provider will do blood tests before and during your treatment with Iclusig to check for liver problems. Get medical help right away if you get any of these symptoms of liver problems during treatment:- yellowing of your skin or the white part of your eyes (jaundice)
- dark "tea-colored" urine
- sleepiness
- loss of appetite
- bleeding or bruising
What is Iclusig?
Iclusig is a prescription medicine used to treat adults who have:- chronic phase, accelerated phase, or blast phase chronic myeloid leukemia (CML) or Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ ALL) who cannot receive any other tyrosine kinase inhibitor (TKI) medicines
- a specific type of abnormal gene (T315I-positive) chronic phase, accelerated phase, or blast phase CML, or T315I-positive Ph+ ALL
It is not known if Iclusig is safe and effective in children less than 18 years of age.Before you take Iclusig, tell your healthcare provider about all of your medical conditions, including if you: - have a history of blood clots in your blood vessels (arteries or veins)
- have heart problems, including heart failure, irregular heartbeats, and QT prolongation
- have diabetes
- have a history of high cholesterol
- have liver problems
- have had inflammation of your pancreas (pancreatitis)
- have high blood pressure
- have bleeding problems
- plan to have surgery or have had a recent surgery. You should stop taking Iclusig at least 1 week before planned surgery. See "What are the possible side effects of Iclusig?".
- are lactose (milk sugar) intolerant. Iclusig tablets contain lactose.
- drink grapefruit juice
- are pregnant or plan to become pregnant. Iclusig can harm your unborn baby.
- Your healthcare provider will do a pregnancy test before you start taking Iclusig.
- You should not become pregnant during treatment with Iclusig.
-
For females who can become pregnant:
- Use an effective form of birth control during treatment and for 3 weeks after your last dose of Iclusig.
- Tell your healthcare provider right away if you become pregnant or think you might be pregnant during treatment with Iclusig.
- Iclusig may affect your ability to have children. Tell your healthcare provider if this is a concern for you.
- are breastfeeding or plan to breastfeed. It is not known if Iclusig passes into your breast milk. Do not breastfeed during treatment and for 6 days after your last dose of Iclusig.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.How should I take Iclusig? - Take Iclusig exactly as your healthcare provider tells you to take it.
- Swallow Iclusig tablets whole. Do not crush or dissolve Iclusig tablets.
- You may take Iclusig with or without food.
- If you miss a dose of Iclusig, take your next dose at your regular time. Do not take 2 doses at the same time to make up for a missed dose.
- If you take too much Iclusig, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of Iclusig?
Iclusig may cause serious side effects, including:- See "What is the most important information I should know about Iclusig?".
- High blood pressure. Your blood pressure should be checked regularly and any high blood pressure should be treated while you are taking Iclusig. Tell your healthcare provider if you get confusion, headaches, dizziness, chest pain or shortness of breath.
- Inflammation of the pancreas (pancreatitis). Tell your healthcare provider if you get any of the following symptoms: sudden stomach-area pain or discomfort, nausea, and vomiting. Your healthcare provider should do blood tests to check for pancreatitis during treatment with Iclusig.
-
Neuropathy. Iclusig may cause damage to the nerves in your arms, brain, hands, legs, or feet (neuropathy). Tell your healthcare provider if you get any of these symptoms during treatment with Iclusig:
- muscle weakness, tingling, burning, pain, and loss of feeling in your hands and feet
- double vision and other problems with eyesight, trouble moving the eye, drooping of part of the face, sagging or drooping eyelids, and change in taste
- Effects on the eye. Serious eye problems that can lead to blindness or blurred vision may happen with Iclusig. Tell your healthcare provider if you get any of the following symptoms: bleeding in the eye, perceived flashes of light, light sensitivity, floaters, dry inflamed, swollen, or itchy eyes, and eye pain. Your healthcare provider will monitor your vision before and during your treatment with Iclusig.
- Severe bleeding. Iclusig can cause bleeding which can be serious and may lead to death. Tell your healthcare provider if you get any signs of bleeding during treatment with Iclusig including:
- vomiting blood or if your vomit looks like coffee-grounds
- pink or brown urine
- red or black (looks like tar) stools
- coughing up blood or blood clots
- unusual bleeding or bruising of your skin
- menstrual bleeding that is heavier than normal
- unusual vaginal bleeding
- nose bleeds that happen often
- drowsiness or difficulty being awakened
- confusion
- headache
- change in speech
-
Fluid retention. Your body may hold too much fluid (fluid retention). Tell your healthcare provider right away if you get any of these symptoms during treatment with Iclusig:
- swelling of your hands, ankles, feet, face, or all over your body
- weight gain
- shortness of breath and cough
- Irregular heartbeat. Iclusig may cause an irregular heartbeat. Tell your healthcare provider right away if you experience loss of consciousness, fainting, dizziness, chest pain or palpitations.
- Low blood cell counts. Iclusig may cause low blood cell counts, which can be severe. Your healthcare provider will check your blood counts regularly during treatment with Iclusig. Tell your healthcare provider right away if you have a fever or any signs of an infection while taking Iclusig.
-
Tumor Lysis Syndrome (TLS). TLS is caused by a fast breakdown of cancer cells. TLS can cause you to have:
- kidney failure and the need for dialysis treatment
- an abnormal heartbeat
- Reversible Posterior Leukoencephalopathy Syndrome (RPLS – also known as Posterior Reversible Encephalopathy Syndrome – PRES). Iclusig may trigger a condition called RPLS. Call your healthcare provider right away if you get headaches, seizures, confusion, changes in vision or problems thinking.
-
Wound healing problems. Wound healing problems have happened in some people who take Iclusig. Tell your healthcare provider if you plan to have any surgery before or during treatment with Iclusig.
- You should stop taking Iclusig at least 1 week before planned surgery.
- Your healthcare provider should tell you when you may start taking Iclusig again after surgery.
-
A tear in your stomach or intestinal wall (perforation). Tell your healthcare provider right away if you get:
- severe pain in your stomach-area (abdomen)
- swelling of the abdomen
- high fever
- stomach-area (abdomen) pain
- skin rash
- constipation
- headache
- dry skin
- blood clots or blockage in blood vessels (arteries)
- tiredness
- high blood pressure
- fever
- joint pain
- nausea
- diarrhea
- increase in lipase levels (a blood test done to check your pancreas)
- vomiting
- muscle pain
- pain in arms, hands, legs, and feet
Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with Iclusig if you have certain side effects.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of Iclusig. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store Iclusig?
Store Iclusig at room temperature between 68°F to 77°F (20°C to 25°C).
Keep Iclusig and all medicines out of the reach of children.General information about the safe and effective use of Iclusig
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Iclusig for a condition for which it was not prescribed. Do not give Iclusig to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about Iclusig that is written for health professionals.What are the ingredients in Iclusig?
Active ingredient: ponatinib
Inactive ingredients: lactose monohydrate, microcrystalline cellulose, sodium starch glycolate (type B), colloidal silicon dioxide and magnesium stearate. The tablet coating consists of talc, polyethylene glycol, polyvinyl alcohol and titanium dioxide.
For more information, go to www.iclusig.com or call 1-844-817-6468.
Manufactured for: Takeda Pharmaceutical Company Limited. Iclusig® is a registered trademark of ARIAD Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, 40 Landsdowne Street Cambridge, MA 02139-4234
©2012 - 2020 ARIAD Pharmaceuticals, Inc., All rights reserved. ICL348 R3 -
PRINCIPAL DISPLAY PANEL - 15 mg Tablet Bottle Label
NDC: 63020-535-30
ICLUSIG®
(ponatinib) tablets15 mg
Rx onlyEach tablet contains ponatinib HCl
equivalent to 15 mg ponatinibDispense Attached Medication Guide
30 tablets
Takeda Logo

-
PRINCIPAL DISPLAY PANEL - 45 mg Tablet Bottle Label
NDC: 63020-534-30
ICLUSIG®
(ponatinib) tablets45 mg
Rx onlyEach tablet contains ponatinib HCl
equivalent to 45 mg ponatinibDispense Attached Medication Guide
30 tablets
Takeda Logo

-
PRINCIPAL DISPLAY PANEL - 30 mg Tablet Bottle Label
NDC: 63020-533-30
ICLUSIG®
(ponatinib) tablets30 mg
Rx onlyEach tablet contains ponatinib HCl
equivalent to 30 mg ponatinibDispense Attached Medication Guide
30 tablets
Takeda Logo
-
INGREDIENTS AND APPEARANCE
ICLUSIG
ponatinib hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63020-535 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ponatinib hydrochloride (UNII: 96R6PU3D8J) (ponatinib - UNII:4340891KFS) ponatinib 15 mg Inactive Ingredients Ingredient Name Strength lactose monohydrate (UNII: EWQ57Q8I5X) microcrystalline cellulose (UNII: OP1R32D61U) sodium starch glycolate type B potato (UNII: 27NA468985) silicon dioxide (UNII: ETJ7Z6XBU4) magnesium stearate (UNII: 70097M6I30) Product Characteristics Color WHITE Score no score Shape ROUND Size 6mm Flavor Imprint Code A5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63020-535-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 12/14/2012 2 NDC: 63020-535-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 12/14/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203469 12/14/2012 ICLUSIG
ponatinib hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63020-534 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ponatinib hydrochloride (UNII: 96R6PU3D8J) (ponatinib - UNII:4340891KFS) ponatinib 45 mg Inactive Ingredients Ingredient Name Strength lactose monohydrate (UNII: EWQ57Q8I5X) microcrystalline cellulose (UNII: OP1R32D61U) sodium starch glycolate type B potato (UNII: 27NA468985) silicon dioxide (UNII: ETJ7Z6XBU4) magnesium stearate (UNII: 70097M6I30) Product Characteristics Color WHITE Score no score Shape ROUND Size 10mm Flavor Imprint Code AP4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63020-534-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 12/14/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203469 12/14/2012 ICLUSIG
ponatinib hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63020-533 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ponatinib hydrochloride (UNII: 96R6PU3D8J) (ponatinib - UNII:4340891KFS) ponatinib 30 mg Inactive Ingredients Ingredient Name Strength lactose monohydrate (UNII: EWQ57Q8I5X) microcrystalline cellulose (UNII: OP1R32D61U) sodium starch glycolate type B potato (UNII: 27NA468985) silicon dioxide (UNII: ETJ7Z6XBU4) magnesium stearate (UNII: 70097M6I30) Product Characteristics Color WHITE Score no score Shape ROUND Size 8mm Flavor Imprint Code C7 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63020-533-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 04/22/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA203469 04/22/2015 Labeler - Millennium Pharmaceuticals, Inc. (804148757)
Trademark Results [Iclusig]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ICLUSIG 85254659 4324899 Live/Registered |
ARIAD Pharmaceuticals, Inc. 2011-03-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.