OSELTAMIVIR PHOSPHATE for suspension
Oseltamivir phosphate by
Drug Labeling and Warnings
Oseltamivir phosphate by is a Prescription medication manufactured, distributed, or labeled by Preferred Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use OSELTAMIVIR PHOSPHATE FOR ORAL SUSPENSION safely and effectively. See full prescribing information for OSELTAMIVIR PHOSPHATE FOR ORAL SUSPENSION.
OSELTAMIVIR PHOSPHATE for oral suspension
Initial U.S. Approval: 1999
INDICATIONS AND USAGE
Oseltamivir phosphate for oral suspension is an influenza neuraminidase inhibitor (NAI) indicated for: (1)
- Treatment of acute, uncomplicated influenza A and B in patients 2 weeks of age and older who have been symptomatic for no more than 48 hours. ()
- Prophylaxis of influenza A and B in patients 1 year and older. ()
Limitations of Use: (1)
- Not a substitute for annual influenza vaccination. ()
- Consider available information on influenza drug susceptibility patterns and treatment effects when deciding whether to use. ()
- Not recommended for patients with end-stage renal disease not undergoing dialysis. ()
DOSAGE AND ADMINISTRATION
Treatment of influenza (2)
- Adults and adolescents (13 years and older): 75 mg twice daily for 5 days ()
- Pediatric patients 1 to 12 years of age: Based on weight twice daily for 5 days ()
- Pediatric patients 2 weeks to less than 1 year of age: 3mg/kg twice daily for 5 days ()
- Renally impaired adult patients (creatinine clearance greater than 30 to 60 mL/min): Reduce to 30 mg twice daily for 5 days ()
- Renally impaired adult patients (creatinine clearance greater than 10 to 30 mL/min): Reduce to 30 mg once daily for 5 days ()
- ESRD patients on hemodialysis: Reduce to 30 mg immediately and then 30 mg after every hemodialysis cycle. Treatment duration not to exceed 5 days ()
- ESRD patients on CAPD: Reduce to a single 30 mg dose immediately ()
Prophylaxis of influenza (2)
- Adults and adolescents (13 years and older): 75 mg once daily for at least 10 days ()
- Community outbreak: 75 mg once daily for up to 6 weeks () (2)
- Pediatric patients 1 to 12 years of age: Based on weight once daily for 10 days ()
- Community outbreak: Based on weight once daily for up to 6 weeks () (2)
- Renally impaired adult patients (creatinine clearance greater than 30 to 60 mL/min): Reduce to 30 mg once daily ()
- Renally impaired adult patients (creatinine clearance greater than 10 to 30 mL/min): Reduce to 30 mg once every other day ()
- ESRD patients on hemodialysis: Reduce to 30 mg immediately and then 30 mg after alternate hemodialysis cycles for the recommended duration of prophylaxis ()
- ESRD patients on CAPD: Reduce to 30 mg immediately and then 30 mg once weekly for the recommended duration of prophylaxis ()
DOSAGE FORMS AND STRENGTHS
For oral suspension: 360 mg oseltamivir base supplied as powder (constituted to a final concentration of 6 mg/mL) (3)
CONTRAINDICATIONS
Patients with known serious hypersensitivity to oseltamivir or any of the components of oseltamivir phosphate for oral suspension (4)
WARNINGS AND PRECAUTIONS
- Serious skin/hypersensitivity reactions such as Stevens-Johnson Syndrome, toxic epidermal necrolysis and erythema multiforme: Discontinue oseltamivir phosphate for oral suspension and initiate appropriate treatment if allergic-like reactions occur or are suspected. (5.1)
- Neuropsychiatric events: Patients with influenza, including those receiving oseltamivir phosphate for oral suspension, particularly pediatric patients, may be at an increased risk of confusion or abnormal behavior early in their illness. Monitor for signs of abnormal behavior. (5.2)
ADVERSE REACTIONS
Most common adverse reactions (greater than 1 % and more common than with placebo):
- Treatment studies – Nausea, vomiting, headache. (6.1)
- Prophylaxis studies – Nausea, vomiting, headache, pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Ajanta Pharma USA, Inc., at 1-855-664-7744 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Live attenuated influenza vaccine (LAIV), intranasal:
Avoid administration of LAIV within 2 weeks before or 48 hours after oseltamivir phosphate for oral suspension use, unless medically indicated. (7)
See 17 for PATIENT COUNSELING INFORMATION, FDA-approved patient labeling and FDA-approved patient labeling.
Revised: 8/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Treatment of Influenza
1.2 Prophylaxis of Influenza
1.3 Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Dosage and Administration Overview
2.2 Recommended Dosage for Treatment of Influenza
2.3 Recommended Dosage for Prophylaxis of Influenza
2.4 Dosage in Patients with Renal Impairment
2.5 Preparation and Storage of Constituted Oseltamivir Phosphate for Oral Suspension
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Skin/Hypersensitivity Reactions
5.2 Neuropsychiatric Events
5.3 Risk of Bacterial Infections
5.4 Fructose Intolerance in Patients with Hereditary Fructose Intolerance
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Influenza Vaccines
7.2 Drugs Without Clinically Significant Drug Interaction with Oseltamivir Phosphate for Oral Suspension
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
8.8 Use in Patients with Chronic Conditions
8.9 Immunocompromised Patients
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Treatment of Influenza
14.2 Prophylaxis of Influenza
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Treatment of Influenza
Oseltamivir phosphate for oral suspension is indicated for the treatment of acute, uncomplicated illness due to influenza A and B infection in patients 2 weeks of age and older who have been symptomatic for no more than 48 hours.
1.2 Prophylaxis of Influenza
Oseltamivir phosphate for oral suspension is indicated for the prophylaxis of influenza A and B in patients 1 year and older.
1.3 Limitations of Use
- Oseltamivir phosphate for oral suspension is not a substitute for early influenza vaccination on an annual basis as recommended by the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices.
- Influenza viruses change over time. Emergence of resistance substitutions could decrease drug effectiveness. Other factors (for example, changes in viral virulence) might also diminish clinical benefit of antiviral drugs. Prescribers should consider available information on influenza drug susceptibility patterns and treatment effects when deciding whether to use oseltamivir phosphate for oral suspension [see Microbiology ()].
- Oseltamivir phosphate for oral suspension is not recommended for patients with end-stage renal disease not undergoing dialysis [see Dosage and Administration () and Use in Specific Populations ()].
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage and Administration Overview
Administer oseltamivir phosphate for oral suspension for the treatment of influenza in patients 2 weeks of age or older [see Dosage and Administration ()] or for prophylaxis of influenza in patients 1 year and older [see Dosage and Administration ()] using:
- Oseltamivir phosphate for oral suspension (supplied as a powder). This is the preferred formulation (6 mg per mL) for patients who cannot swallow capsules. Prior to use, the supplied oseltamivir phosphate for oral suspension powder must be constituted with water by the pharmacist to produce the oral suspension [see Dosage and Administration ()].
The oral suspension may be taken with or without food; however, tolerability may be enhanced if oseltamivir phosphate for oral suspension is taken with food.
Adjust the oseltamivir phosphate for oral suspension dosage in patients with moderate or severe renal impairment [see Dosage and Administration ()].
2.2 Recommended Dosage for Treatment of Influenza
Initiate treatment with oseltamivir phosphate for oral suspension within 48 hours of influenza symptom onset.
Adults and Adolescents (13 years of age and older)
The recommended oral dosage of oseltamivir phosphate for oral suspension for treatment of influenza in adults and adolescents 13 years and older is 75 mg twice daily (12.5 mL of oral suspension twice daily) for 5 days.
Pediatric Patients (2 weeks of age through 12 years of age)
Table 1 displays the recommended oral dosage of oseltamivir phosphate for oral suspension for treatment of influenza in pediatric patients 2 weeks of age through 12 years of age and provides information about prescribing the formulation for oral suspension.
2.3 Recommended Dosage for Prophylaxis of Influenza
Initiate post-exposure prophylaxis with oseltamivir phosphate for oral suspension within 48 hours following close contact with an infected individual. Initiate seasonal prophylaxis with oseltamivir phosphate for oral suspension during a community outbreak.
Adults and Adolescents (13 years of age and older)
The recommended dosage of oseltamivir phosphate for oral suspension for prophylaxis of influenza in adults and adolescents 13 years and older is 75 mg orally once daily (12.5 mL of oral suspension once daily) for at least 10 days following close contact with an infected individual and up to 6 weeks during a community outbreak. In immunocompromised patients, oseltamivir phosphate for oral suspension may be continued for up to 12 weeks [see Use in Specific Populations ()]. The duration of protection lasts for as long as oseltamivir phosphate for oral suspension dosing is continued.
Pediatric Patients (1 year to 12 years of age)
Table 1 displays the recommended oral dosage of oseltamivir phosphate for oral suspension for prophylaxis of influenza in pediatric patients 1 year to 12 years of age based on body weight and provides information about prescribing the formulation for oral suspension. Prophylaxis in pediatric patients is recommended for 10 days following close contact with an infected individual and up to 6 weeks during a community outbreak [see Use in Specific Populations (8.4) and Clinical Studies (14.2)].
Table 1: Oseltamivir Phosphate Dosage Recommendations in Pediatric Patients for Treatment and Prophylaxis of Influenza * The recommended duration for post-exposure prophylaxis is 10 days and the recommended duration for community outbreak (seasonal/pre-exposure) prophylaxis is up to 6 weeks (or up to 12 weeks in immunocompromised patients). The amount supplied (e.g., number of bottles) for seasonal prophylaxis may be greater than for post-exposure prophylaxis.
† Use an oral dosing dispensing device that measures the appropriate volume in mL with the oral suspension.
§ For patients less than 1 year of age, provide an appropriate dosing device that can accurately measure and administer small volumes.Weight
Treatment Dosage for 5 days
Prophylaxis Dosage for
10 days*
Volume of Oral Suspension
(6 mg/mL) for each Dose†
Number of Bottles of Oral Suspension to Dispense
Patients from 2 Weeks to less than 1 Year of Age
Any weight
3 mg/kg twice daily
Not applicable
0.5 mL/kg§
1 bottle
Patients 1 to 12 Years of Age Based on Body Weight
15 kg or less
30 mg twice daily
30 mg once daily
5 mL
1 bottle
15.1 kg to 23 kg
45 mg twice daily
45 mg once daily
7.5 mL
2 bottles
23.1 kg to 40 kg
60 mg twice daily
60 mg once daily
10 mL
2 bottles
40.1 kg or more
75 mg twice daily
75 mg once daily
12.5 mL
3 bottles
2.4 Dosage in Patients with Renal Impairment
Table 2 displays the dosage recommendations for the treatment and prophylaxis of influenza in adults with various stages of renal impairment (estimated creatinine clearance of less than or equal to 90 mL per minute). Dosage modifications are recommended in adults with an estimated creatinine clearance less than or equal to 60 mL per minute [see Use in Specific Population () and Clinical Pharmacology ()].
Table 2: Recommended Dosage Modifications for Treatment and Prophylaxis of Influenza in Adults with Renal Impairment or End Stage Renal Disease (ESRD) on Dialysis * Oral suspension can be used for 30 mg dosing.
† The recommended duration for post-exposure prophylaxis is at least 10 days and the recommended duration for community outbreak (seasonal/pre-exposure) prophylaxis is up to 6 weeks (or up to 12 weeks in immunocompromised patients). ‡ Data derived from studies in continuous ambulatory peritoneal dialysis (CAPD) patients.
Renal Impairment (Creatinine Clearance)
Recommended Treatment Regimen*
Recommended Prophylaxis Regimen*†
Mild
(>60-90 mL/minute)
75 mg twice daily for 5 days
75 mg once daily
Moderate
(>30-60 mL/minute)
30 mg twice daily for 5 days
30 mg once daily
Severe
(>10-30 mL/minute)
30 mg once daily for 5 days
30 mg every other day
ESRD Patients on Hemodialysis
(≤10 mL/minute)
30 mg immediately and then 30 mg after every hemodialysis cycle (treatment duration not to exceed 5 days)
30 mg immediately and then 30 mg after alternate hemodialysis cycles
ESRD Patients on Continuous Ambulatory Peritoneal Dialysis‡
(≤10 mL/minute)
A single 30 mg dose administered immediately
30 mg immediately and then 30 mg once weekly
ESRD Patients not on Dialysis
Oseltamivir Phosphate for oral suspension is not recommended
Oseltamivir Phosphatefor oral suspension is not recommended
2.5 Preparation and Storage of Constituted Oseltamivir Phosphate for Oral Suspension
Prior to dispensing to the patient, constitute Oseltamivir phosphate for oral suspension (supplied as powder):
a) Tap the closed bottle containing the supplied Oseltamivir phosphate for oral suspension white powder several times to loosen the powder.
b) Measure 55 mL of water in a graduated cylinder.
c) Add the total amount of water for constitution to the bottle.
d) Close bottle with child-resistant cap tightly and shake the closed bottle well for 15 seconds.
e) Label the bottle with instructions to “Shake Well Before Use”.
f) The constituted oral suspension contains 360 mg of oseltamivir base per 60 mL of volume (6 mg per mL) and is white, tutti-frutti–flavored). Use the constituted oral suspension within 17 days of preparation when stored under refrigeration, 2º to 8ºC (36º to 46ºF), or within 10 days if stored at controlled room temperature, 25ºC (77ºF). Write the expiration date of the constituted oral suspension on the bottle label.
g) Ensure patients have an oral dosing dispenser that measures the appropriate volume in milliliters. Counsel patients on how to utilize the oral dosing dispenser and correctly measure the oral suspension as prescribed (see Tables 1 and 2).
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Oseltamivir Phosphate for Oral Suspension is contraindicated in patients with known serious hypersensitivity to oseltamivir or any component of the product. Severe allergic reactions have included anaphylaxis and serious skin reactions including toxic epidermal necrolysis, Stevens - Johnson syndrome, and erythema multiforme [see Warnings and Precautions ()].
-
5 WARNINGS AND PRECAUTIONS
5.1 Serious Skin/Hypersensitivity Reactions
Cases of anaphylaxis and serious skin reactions including toxic epidermal necrolysis, Stevens - Johnson syndrome, and erythema multiforme have been reported in postmarketing experience with oseltamivir phosphate for oral suspension. Stop oseltamivir phosphate for oral suspension and institute appropriate treatment if an allergic-like reaction occurs or is suspected. The use of oseltamivir phosphate for oral suspension is contraindicated in patients with known serious hypersensitivity to oseltamivir phosphate for oral suspension [see Contraindications () and Adverse Reactions ()].
5.2 Neuropsychiatric Events
There have been postmarketing reports of delirium and abnormal behavior leading to injury, and in some cases resulting in fatal outcomes, in patients with influenza who were receiving oseltamivir phosphate for oral suspension [see Adverse Reactions ()]. Because these events were reported voluntarily during clinical practice, estimates of frequency cannot be made but they appear to be uncommon based on oseltamivir phosphate for oral suspension usage data. These events were reported primarily among pediatric patients and often had an abrupt onset and rapid resolution. The contribution of oseltamivir phosphate for oral suspension to these events has not been established. Influenza can be associated with a variety of neurologic and behavioral symptoms that can include events such as hallucinations, delirium, and abnormal behavior, in some cases resulting in fatal outcomes. These events may occur in the setting of encephalitis or encephalopathy but can occur without obvious severe disease. Closely monitor oseltamivir phosphate for oral suspension-treated patients with influenza for signs of abnormal behavior. If neuropsychiatric symptoms occur, evaluate the risks and benefits of continuing oseltamivir phosphate for oral suspension for each patient.
5.3 Risk of Bacterial Infections
There is no evidence for efficacy of oseltamivir phosphate for oral suspension in any illness caused by pathogens other than influenza viruses. Serious bacterial infections may begin with influenza-like symptoms or may coexist with or occur as complications during the course of influenza. Oseltamivir phosphate for oral suspension has not been shown to prevent such complications. Prescribers should be alert to the potential for secondary bacterial infections and treat them as appropriate.
5.4 Fructose Intolerance in Patients with Hereditary Fructose Intolerance
Fructose can be harmful to patients with hereditary fructose intolerance. One dose of 75 mg oseltamivir phosphate for oral suspension delivers 2 grams of sorbitol. This is above the daily maximum limit of sorbitol for patients with hereditary fructose intolerance, and may cause dyspepsia and diarrhea.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed below and elsewhere in the labeling:
- Serious skin and hypersensitivity reactions [see Warnings and Precautions (]
- Neuropsychiatric events [see Warnings and Precautions (]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions from Treatment and Prophylaxis Trials in Adult and Adolescent Subjects (13 years of age and older)
The overall safety profile of oseltamivir phosphate is based on data from 2,646 adult and adolescent subjects that received the recommended dosage of 75 mg orally twice daily for 5 days for treatment of influenza and 1,943 adult and adolescent subjects that received the recommended dosage of 75 mg orally once daily for up to 6 weeks for prophylaxis of influenza in clinical trials.
The most common adverse reactions in the pooled treatment and pooled prophylaxis trials in adults and adolescents are displayed in Table 5. The majority of these adverse reactions were reported on a single occasion, occurred on either the first or second treatment day and resolved spontaneously within 1 to 2 days. This summary includes otherwise healthy adults/adolescents and subjects "at risk" (subjects at higher risk of developing complications associated with influenza, e.g., elderly patients and patients with chronic cardiac or respiratory disease). In general, the safety profile in the subjects "at risk" was qualitatively similar to that in otherwise healthy adults/adolescents.
Table 3: Adverse Reactions Occurring in greater than or equal to 1 % of Adults and Adolescents (13 years of age and older) in Treatment and Prophylaxis Trials * * Adverse reactions that occurred in greater than or equal to1% of oseltamivir phosphate-treated adults and adolescents and greater than or equal to 1% greater in oseltamivir phosphate-treated subjects compared to placebo-treated subjects in either the treatment or prophylaxis trials. System Organ Class
Adverse Reaction
Treatment Trials
Prophylaxis Trials
Oseltamivir phosphate
75 mg twice daily
(n = 2,646)
Placebo
(n = 1,977)
Oseltamivir phosphate
75 mg once daily
(n = 1,943)
Placebo
(n = 1,586)
Gastrointestinal Disorders
Nausea
10%
6%
8%
4%
Vomiting
8%
3%
2%
1%
Nervous System Disorders
Headache
2%
1%
17%
16%
General Disorders
Pain
<1%
<1%
4%
3%
Adverse Reactions from Treatment and Prophylaxis Trials in Pediatric Subjects (1 year to 12 years of age)
A total of 1,481 pediatric subjects (including otherwise healthy pediatric subjects aged 1 year to 12 years and asthmatic pediatric subjects aged 6 to 12 years) participated in clinical trials of oseltamivir phosphate for the treatment of influenza. A total of 859 pediatric subjects received treatment with oseltamivir phosphate for oral suspension either at a 2 mg per kg twice daily for 5 days or weight-band dosing. Vomiting was the only adverse reaction reported at a frequency of greater than 1% in subjects receiving Oseltamivir phosphate (16%) compared to placebo (8%).
Amongst the 148 pediatric subjects aged 1 year to 12 years who received oseltamivir phosphate at doses of 30 mg to 60 mg once daily for 10 days in a post-exposure prophylaxis study in household contacts (n = 99), and in a separate 6–week seasonal influenza prophylaxis safety study (n = 49), vomiting was the most frequent adverse reaction (8% on oseltamivir phosphate versus 2% in the no prophylaxis group).
Adverse Reactions from Treatment Trials in Pediatric Subjects (2 weeks to less than 1 year of age)
Assessment of adverse reactions in pediatric subjects 2 weeks to less than 1 year of age was based on two open-label studies that included safety data on 135 influenza-infected subjects 2 weeks to less than 1 year of age (including premature infants at least 36 weeks post conceptional age) exposed to oseltamivir phosphate at doses ranging from 2 mg to 3.5 mg per kg of the formulation for oral suspension twice daily orally for 5 days. The safety profile of oseltamivir phosphate was similar across the age range studied, with vomiting (9%), diarrhea (7%) and diaper rash (7%) being the most frequently reported adverse reactions, and was generally comparable to that observed in older pediatric and adult subjects.
Adverse Reactions from the Prophylaxis Trial in Immunocompromised Subjects
In a 12-week seasonal prophylaxis study in 475 immunocompromised subjects, including 18 pediatric subjects 1 year to 12 years of age, the safety profile in the 238 subjects receiving oseltamivir phosphate 75 mg once daily was consistent with that previously observed in other oseltamivir phosphate prophylaxis clinical trials [see Clinical Studies (14.2)].
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of oseltamivir phosphate. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to oseltamivir phosphate exposure.
General disorders and administration site conditions: Swelling of the face or tongue, allergy, anaphylactic/anaphylactoid reactions, hypothermia
Skin and subcutaneous tissue disorders: Rash, dermatitis, urticaria, eczema, toxic epidermal necrolysis, Stevens-Johnson Syndrome, erythema multiforme [see Warnings and Precautions (]
Gastrointestinal Disorders: Gastrointestinal bleeding, hemorrhagic colitis
Cardiac Disorders: Arrhythmia
Hepatobiliary Disorders: Hepatitis, abnormal liver function tests
Nervous System Disorders: Seizure
Metabolism and Nutrition Disorders: Aggravation of diabetes
Psychiatric Disorders: Abnormal behavior, delirium, including symptoms such as hallucinations, agitation, anxiety, altered level of consciousness, confusion, nightmares, delusions [see Warnings and Precautions ()]
-
7 DRUG INTERACTIONS
7.1 Influenza Vaccines
Live Attenuated Influenza Vaccine
The concurrent use of oseltamivir phosphate with live attenuated influenza vaccine (LAIV) intranasal has not been evaluated. However, because of the potential for oseltamivir phosphate to inhibit replication of live vaccine virus and possibly reduce the efficacy of LAIV, avoid administration of LAIV within 2 weeks before or 48 hours after oseltamivir phosphate administration, unless medically indicated.
Inactivated Influenza Vaccine
Inactivated influenza vaccine can be administered at any time relative to use of oseltamivir phosphate.
7.2 Drugs Without Clinically Significant Drug Interaction with Oseltamivir Phosphate for Oral Suspension
No dose adjustments are needed for either oseltamivir or the concomitant drug when coadministering oseltamivir with amoxicillin, acetaminophen, aspirin, cimetidine, antacids (magnesium and aluminum hydroxides and calcium carbonates), rimantadine, amantadine, or warfarin [see Clinical Pharmacology ()]
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies with oseltamivir phosphate in pregnant women to inform a drug-associated risk of adverse developmental outcomes. Available published epidemiological data suggest that oseltamivir phosphate, taken in any trimester, is not associated with an increased risk of birth defects. However, these studies individually are limited by small sample sizes, use of different comparison groups, and some lacked information on dose, which preclude a definitive assessment of the risk [see Data and Clinical Pharmacology ()]. In animal reproduction studies with oseltamivir, no adverse developmental effects were observed at clinically relevant exposures (see Data).The background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage is 2% to 4% and 15% to 20%, respectively.
Clinical ConsiderationsDisease-Associated Maternal and/or Embryo/Fetal Risk
Pregnant women are at higher risk of severe complications from influenza, which may lead to adverse pregnancy and/or fetal outcomes including maternal death, still births, birth defects, preterm delivery, low birth weight and small for gestational age.
Data
Human Data
Published prospective and retrospective observational studies of more than 5,000 women exposed to oseltamivir during pregnancy, including more than 1,000 women exposed in the first trimester, suggest that the observed rate of congenital malformations was not increased above the rate in the general comparison population, regardless of when therapy was administered during the gestational period. However, individually, none of these studies had adequate sample sizes and some lacked information on dose, which preclude a definitive assessment of the risk.
Animal Data
Oseltamivir was administered orally during organogenesis to pregnant rats (at 50 mg/kg/day, 250 mg/kg/day, or 1500 mg/kg/day on gestation days 6 to 17) and rabbits (at 50 mg/kg/day, 150 mg/kg/day, or 500 mg/kg/day on gestation days 6 to 18). In rats, embryo-fetal effects consisting of an increased incidence of minor skeletal malformations were observed at a maternally toxic dose (1500 mg/kg/day), resulting in systemic drug exposures (based on AUC for oseltamivir carboxylate) 190 times human exposures at the maximum recommended human dose (MRHD) of oseltamivir phosphate (75 mg twice a day). In the rabbit study, embryo-fetal effects consisting of an increased incidence of minor skeletal abnormalities and variants were observed at maternally toxic doses (greater than or equal to150 mg/kg/day) resulting in systemic exposures (based on AUC for oseltamivir carboxylate) greater than or equal to 8 times human exposures at the MRHD of oseltamivir phosphate.
In prenatal and postnatal development studies in rats, oseltamivir was administered orally (at 50 mg/kg/day, 250 mg/kg/day, 500 mg/kg/day, or 1500 mg/kg/day) from organogenesis through late gestation, delivery, and lactation (gestation day 6 to postpartum/lactation day 20). Prolonged parturition duration and reduced offspring viability were observed at a maternally toxic dose (1500 mg/kg/day). No adverse maternal or offspring effects were observed at doses less than or equal to 500 mg/kg/day, resulting in systemic drug exposures (based on AUC for oseltamivir carboxylate) 44 times human exposures at the MRHD of oseltamivir phosphate.
8.2 Lactation
Risk Summary
Based on limited published data, oseltamivir and oseltamivir carboxylate have been shown to be present in human milk at low levels considered unlikely to lead to toxicity in the breastfed infant. Postmarketing experience has not reported any information to suggest serious adverse effects of oseltamivir exposure via breast milk in infants. It is not known if oseltamivir affects human milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for oseltamivir phosphate and any potential adverse effects on the breastfed child from the drug or from the underlying maternal condition.8.4 Pediatric Use
Treatment of Influenza
The safety and efficacy of oseltamivir phosphate for the treatment of influenza in pediatric patients 2 weeks old to 17 years of age has been established [see Dosage and Administration (), Clinical Pharmacology (), and Clinical Studies ()] and is based on:
- 13 to 17 years of age: Safety and efficacy in adolescent patients 13 to 17 years of age was supported by adequate and well-controlled trials in adults and adolescents and younger pediatric patients and safety data in adolescents treated with oseltamivir phosphate in a study of treatment and prophylaxis.
- 1 year to 12 years of age: Safety and efficacy in pediatric patients 1 year to 12 years of age was supported by results of one double-blind, placebo-controlled trial in 452 pediatric patients with influenza in whom oseltamivir phosphate 2 mg per kg twice daily or placebo was administered within 48 hours of symptom onset [see Clinical Studies ()]. Additional safety information was provided in a double-blind, placebo-controlled trial in pediatric patients 6 to 12 years of age with known asthma. Efficacy could not be established in pediatric patients with asthma.
- 2 weeks to less than 1 year of age: Safety and efficacy in pediatric patients 2 weeks to less than 1 year of age is supported by adequate and well-controlled trials in adults and older pediatric patients and two open-label trials of oseltamivir phosphate (2 mg per kg to 3.5 mg per kg twice daily for 5 days) in 136 pediatric subjects 2 weeks to less than 1 year of age. In these two trials, the oseltamivir plasma concentrations in these subjects were similar to or higher than the oseltamivir plasma concentrations observed in older pediatric subjects and adults [see Clinical Pharmacology () and Clinical Studies ()].
The safety and efficacy of oseltamivir phosphate for treatment of influenza in pediatric patients less than 2 weeks of age have not been established.
Prophylaxis of Influenza
The safety and efficacy of oseltamivir phosphate for the prophylaxis of influenza in pediatric patients 1 year to 17 years old has been established [see Dosage and Administration (), Clinical Pharmacology (), and Clinical Studies (14.2)] and is based on:
- 13 to 17 years of age: Prophylaxis in adolescent patients 13 to 17 years of age is supported by one randomized, placebo-controlled post-exposure household prophylaxis trial of oseltamivir phosphate 75 mg taken orally once daily for 7 days in household contacts including 207 adolescents [see Clinical Studies (14.2)].
- 1 year to 12 years of age: oseltamivir phosphate for prophylaxis in pediatric patients 1 year to 12 years of age is supported by one randomized, open-label, post-exposure household prophylaxis trial including pediatric subjects 1 year to 12 years of age who received 30 to 60 mg of oseltamivir phosphate for oral suspension (supplied as powder) taken orally once daily for 10 days [see Clinical Studies (14.2)]. Additional safety information was provided in a 6-week seasonal prophylaxis (community outbreak) safety study in 49 patients 1 year to 12 years of age.
The safety and efficacy of oseltamivir phosphate for prophylaxis of influenza have not been established for pediatric patients less than 1 year of age.
8.5 Geriatric Use
Treatment of Influenza
Of the 4,765 adults in clinical trials of oseltamivir phosphate for the treatment of influenza, 948 (20%) were 65 years and older, while 329 (7%) were 75 years and older. In three double-blind, placebo-controlled trials in the treatment of influenza in patients at least 65 years old, that enrolled 741 subjects (374 received placebo and 362 received oseltamivir phosphate), no overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger subjects [see Clinical Studies ()].
Prophylaxis of Influenza
Of the 4,603 adults in clinical trials of oseltamivir phosphate for the prophylaxis of influenza, 1,046 (23%) were 65 years and older, while 719 (16%) were 75 years and older. In a randomized, placebo-controlled trial in elderly residents of nursing homes who took oseltamivir phosphate for up to 42 days for the prophylaxis of influenza (Oseltamivir phosphate n=276, placebo n=272), no overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger subjects [see Clinical Studies (14.2)].
8.6 Renal Impairment
Patients with renal impairment had higher blood levels of oseltamivir carboxylate compared to patients with normal renal function which may increase the risk of oseltamivir phosphate-associated adverse reactions. Therefore, dosage adjustment is recommended for patients with a serum creatinine clearance between 10 mL/minute and 60 mL/minute and for patients with end-stage renal disease (ESRD) undergoing routine hemodialysis or continuous peritoneal dialysis treatment [see Dosage and Administration ()]. Oseltamivir phosphate is not recommended for patients with ESRD not undergoing dialysis [see Indications and Usage () and Clinical Pharmacology ()].
8.7 Hepatic Impairment
No dosage adjustment is required in patients with mild to moderate hepatic impairment. The safety and pharmacokinetics in patients with severe hepatic impairment have not been evaluated [see Clinical Pharmacology ()].
8.8 Use in Patients with Chronic Conditions
Efficacy of oseltamivir phosphate in the treatment of influenza in patients with chronic cardiac disease and/or respiratory disease was evaluated in one randomized, placebo-controlled clinical trial. Efficacy in this population, as measured by time to alleviation of all symptoms, was not established, but no new safety signals were identified [see Clinical Studies ()].
No clinical trial data are available regarding treatment of influenza in patients with any medical condition sufficiently severe or unstable to be considered at imminent risk of requiring hospitalization.
8.9 Immunocompromised Patients
Efficacy of oseltamivir phosphate for the treatment or prophylaxis of influenza has not been established in immunocompromised patients [see Clinical Studies (14.2)]. Safety of oseltamivir phosphate has been demonstrated for up to 12 weeks for prophylaxis of influenza in immunocompromised patients [see Adverse Reactions ()].
-
10 OVERDOSAGE
Reports of overdoses with oseltamivir phosphate for oral suspension have been received from clinical trials and during postmarketing experience. In the majority of cases reporting overdose, no adverse reactions were reported. Adverse reactions reported following overdose were similar in nature to those observed with therapeutic doses of oseltamivir phosphate for oral suspension [see Adverse Reactions (6)].
-
11 DESCRIPTION
Oseltamivir phosphate, USP an influenza neuraminidase inhibitor (NAI), is available as:
- A powder for oral suspension, which when constituted with water as directed contains 6 mg per mL oseltamivir base.
In addition to the active ingredient, the powder for oral suspension contains trisodium citrate, saccharin sodium, sodium benzoate, sorbitol, titanium dioxide, tutti-frutti flavoring, and xanthan gum.
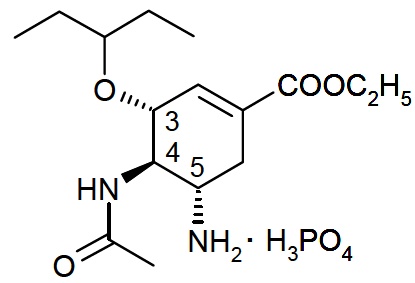
Oseltamivir phosphate, USP is a white to off-white powder with the chemical name (3R,4R,5S)-4-acetylamino-5-amino-3(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid, ethyl ester, phosphate (1:1). The chemical formula is C16H28N2O4 (free base). The molecular weight is 312.4 for oseltamivir free base and 410.4 for oseltamivir phosphate salt. The structural formula is as follows:
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Oseltamivir is an antiviral drug with activity against influenza virus [see Microbiology ()].
12.3 Pharmacokinetics
Absorption and Bioavailability
Oseltamivir is absorbed from the gastrointestinal tract after oral administration of oseltamivir phosphate and is extensively converted predominantly by hepatic esterases to oseltamivir carboxylate. At least 75% of an oral dose reaches the systemic circulation as oseltamivir carboxylate and less than 5% of the oral dose reaches the systemic circulation as oseltamivir (see Table 6).
Table 4: Mean (% CV) Pharmacokinetic Parameters of Oseltamivir and Oseltamivir Carboxylate Following Multiple Dosing of 75 mg Capsules Twice Daily (n=20) Parameter
Oseltamivir
Oseltamivir Carboxylate
Cmax (ng/mL)
65 (26)
348 (18)
AUC0-12h (ng·h/mL)
112 (25)
2719 (20)
Plasma concentrations of oseltamivir carboxylate are proportional to doses up to 500 mg given twice daily (about 6.7 times the maximum recommended oseltamivir phosphate for oral suspension dosage) [see Dosage and Administration (2)]. Coadministration with food had no significant effect on the peak plasma concentration (551 ng/mL under fasted conditions and 441 ng/mL under fed conditions) and the area under the plasma concentration time curve (6,218 ng∙h/mL under fasted conditions and 6,069 ng∙h/mL under fed conditions) of oseltamivir carboxylate.
Distribution
The volume of distribution (Vss) of oseltamivir carboxylate, following intravenous administration in 24 subjects (oseltamivir phosphate is not available as an IV formulation), ranged between 23 and 26 liters.
The binding of oseltamivir carboxylate to human plasma protein is low (3%). The binding of oseltamivir to human plasma protein is 42%, which is insufficient to cause significant displacement-based drug interactions.
Elimination
Absorbed oseltamivir is primarily (greater than 90%) eliminated by conversion to the active metabolite, oseltamivir carboxylate. Plasma concentrations of oseltamivir declined with a half-life of 1 to 3 hours in most subjects after oral administration. Oseltamivir carboxylate is not further metabolized and is eliminated unchanged in urine. Plasma concentrations of oseltamivir carboxylate declined with a half-life of 6 to 10 hours in most subjects after oral administration.
Metabolism
Oseltamivir is extensively converted to the active metabolite, oseltamivir carboxylate, by esterases located predominantly in the liver. Oseltamivir carboxylate is not further metabolized. Neither oseltamivir nor oseltamivir carboxylate is a substrate for, or inhibitor of, cytochrome P450 isoforms.
Excretion
Oseltamivir carboxylate is eliminated entirely (greater than 99%) by renal excretion. Renal clearance (18.8 L/h) exceeds glomerular filtration rate (7.5 L/h), indicating that tubular secretion (via organic anion transporter) occurs in addition to glomerular filtration. Less than 20% of an oral radiolabeled dose is eliminated in feces.
Specific Populations
Renal Impairment
Administration of 100 mg of oseltamivir phosphate twice daily (about 1.3 times the maximum recommended dosage) for 5 days to subjects with various degrees of renal impairment showed that exposure to oseltamivir carboxylate is inversely proportional to declining renal function.
Population-derived pharmacokinetic parameters were determined for patients with varying degrees of renal function including ESRD patients on hemodialysis. Median simulated exposures of oseltamivir carboxylate for recommended treatment and prophylaxis regimens are provided in Table 7. The pharmacokinetics of oseltamivir have not been studied in ESRD patients not undergoing dialysis [see Indications and Usage (), and Use in Specific Populations ()].
Table 5: Simulated Median Treatment Exposure Metrics of Oseltamivir Carboxylate in Patients with Normal Renal Function, with Renal Impairment and ESRD Patients on Hemodialysis * AUC normalized to 48 hours Renal Function/ Impairment
Normal Creatinine Clearance 90-140 mL/min (n=57)
Mild Creatinine Clearance 60-90 mL/min (n=45)
Moderate Creatinine Clearance 30-60 mL/min (n=13)
Severe Creatinine Clearance 10-30 mL/min (n=11)
ESRD Creatinine Clearance <10 mL/min on Hemodialysis (n=24)
Recommended Treatment Regimens
PK exposure parameter
75 mg twice daily
75 mg twice daily
30 mg twice daily
30 mg once daily
30 mg every HD cycle
Cmin (ng/mL)
145
253
180
219
221
Cmax (ng/mL)
298
464
306
477
1170
AUC48 (ng·h/mL)*
11224
18476
12008
16818
23200
Recommended Prophylaxis Regimens
PK exposure parameter
75 mg once daily
75 mg once daily
30 mg once daily
30 mg every other day
30 mg alternate HD cycle
Cmin (ng/mL)
39
62
57
70
42
Cmax (ng/mL)
213
311
209
377
903
AUC48 (ng·hr/mL)*
5294
8336
6262
9317
11200
In continuous ambulatory peritoneal dialysis (CAPD) patients, the peak concentration of oseltamivir carboxylate following a single 30 mg dose of oseltamivir or once weekly oseltamivir was approximately 3-fold higher than in patients with normal renal function who received 75 mg twice daily. The plasma concentration of oseltamivir carboxylate on Day 5 (147 ng/mL) following a single 30 mg dose in CAPD patients is similar to the predicted Cmin (160 ng/mL) in patients with normal renal function following 75 mg twice daily. Administration of 30 mg once weekly to CAPD patients resulted in plasma concentrations of oseltamivir carboxylate at the 168 hour blood sample of 63 ng/mL, which were comparable to the Cmin in patients with normal renal function receiving the approved regimen of 75 mg once daily (40 ng/mL).
Hepatic Impairment
In clinical studies, oseltamivir carboxylate exposure was not altered in subjects with mild or moderate hepatic impairment [see Use in Specific Populations ()].
Pregnant Women
A pooled population pharmacokinetic analysis indicates that the oseltamivir phosphate dosage regimen resulted in lower exposure to the active metabolite in pregnant women (n=59) compared to non-pregnant women (n=33). However, this predicted exposure is expected to have activity against susceptible influenza virus strains and there are insufficient pharmacokinetics and safety data to recommend a dose adjustment for pregnant women [see Use in Specific Populations ()].
Pediatric Subjects (1 year to 12 years of age)
The pharmacokinetics of oseltamivir and oseltamivir carboxylate have been evaluated in a single-dose pharmacokinetic study in pediatric subjects aged 5 to 16 years (n=18) and in a small number of pediatric subjects aged 3 to 12 years (n=5) enrolled in a clinical trial. Younger pediatric subjects cleared both the prodrug and the active metabolite faster than adult subjects resulting in a lower exposure for a given mg/kg dose. For oseltamivir carboxylate, apparent total clearance decreases linearly with increasing age (up to 12 years). The pharmacokinetics of oseltamivir in pediatric subjects over 12 years of age are similar to those in adult subjects [see Use in Specific Populations (8.4)].
Pediatric Subjects (2 weeks to less than 1 year of age)
The pharmacokinetics of oseltamivir and oseltamivir carboxylate have been evaluated in two open-label studies of pediatric subjects less than one year of age (n=122) infected with influenza. Apparent clearance of the active metabolite decreases with decreasing age in subjects less than 1 year of age; however the oseltamivir and oseltamivir carboxylate exposure following a 3 mg/kg dose in subjects under 1 year of age is expected to be within the observed exposures in adults and adolescents receiving 75 mg twice daily and 150 mg twice daily [see Use in Specific Populations (8.4)].
Geriatric Patients
Exposure to oseltamivir carboxylate at steady-state was 25% to 35% higher in geriatric subjects (age range 65 to 78 years) compared to young adults given comparable doses of oseltamivir. Half-lives observed in the geriatric subjects were similar to those seen in young adults. Based on drug exposure and tolerability, dose adjustments are not required for geriatric patients for either treatment or prophylaxis [see Use in Specific Populations ()].
Drug Interaction Studies
Oseltamivir is extensively converted to oseltamivir carboxylate by esterases, located predominantly in the liver. Drug interactions involving competition for esterases have not been extensively reported in literature. Low protein binding of oseltamivir and oseltamivir carboxylate suggests that the probability of drug displacement interactions is low.
In vitro studies demonstrate that neither oseltamivir nor oseltamivir carboxylate is a good substrate for P450 mixed-function oxidases or for glucuronyl transferases.
Coadministration of probenecid results in an approximate two-fold increase in exposure to oseltamivir carboxylate due to a decrease in active anionic tubular secretion in the kidney. However, due to the safety margin of oseltamivir carboxylate, no dose adjustments are required when coadministering with probenecid. No clinically relevant pharmacokinetic interactions have been observed when coadministering oseltamivir with amoxicillin, acetaminophen, aspirin, cimetidine, antacids (magnesium and aluminum hydroxides and calcium carbonates), rimantadine, amantadine, or warfarin.
12.4 Microbiology
Mechanism of Action
Oseltamivir phosphate is an ethyl ester prodrug requiring ester hydrolysis for conversion to the active form, oseltamivir carboxylate. Oseltamivir carboxylate is an inhibitor of influenza virus neuraminidase affecting release of viral particles. The median IC50 values of oseltamivir against influenza A/H1N1, influenza A/H3N2, and influenza B clinical isolates were 2.5 nM (range 0.93 to 4.16 nM, N=74), 0.96 nM (range 0.13 to 7.95 nM, N=774), and 60 nM (20 to 285 nM, N=256), respectively, in a neuraminidase assay with a fluorescently labeled MUNANA substrate.
Antiviral Activity
The antiviral activity of oseltamivir carboxylate against laboratory strains and clinical isolates of influenza virus was determined in cell culture. The concentrations of oseltamivir carboxylate required for inhibition of influenza virus in cell culture were highly variable depending on the assay method used and the virus tested. The 50% and 90% effective concentrations (EC50 and EC90) were in the range of 0.0008 micromolar to greater than 35 micromolar and 0.004 micromolar to greater than 100 micromolar, respectively (1 micromolar=0.284 microgram per mL). The relationship between the antiviral activity in cell culture, inhibitory activity in the neuraminidase assay, and the inhibition of influenza virus replication in humans has not been established.
Resistance
Cell culture studies: Influenza A virus isolates with reduced susceptibility to oseltamivir carboxylate have been recovered by serial passage of virus in cell culture in the presence of increasing concentrations of oseltamivir carboxylate. Reduced susceptibility of influenza virus to inhibition by oseltamivir carboxylate may be conferred by amino acid substitutions in the viral neuraminidase and/or hemagglutinin proteins.
Clinical studies: Reduced susceptibility isolates have been obtained during treatment with oseltamivir and from sampling during community surveillance studies. Changes in the viral neuraminidase that have been associated with reduced susceptibility to oseltamivir carboxylate are summarized in Table 8. The clinical impact of this reduced susceptibility is unknown.
Hemagglutinin (HA) substitutions selected in cell culture and associated with reduced susceptibility to oseltamivir include (influenza virus subtype-specific numbering) A11T, K173E, and R453M in H3N2; and H99Q in influenza B virus (Yamagata lineage). In some cases, HA substitutions were selected in conjunction with known NA resistance substitutions and may contribute to reduced susceptibility to oseltamivir; however, the impact of HA substitutions on antiviral activity of oseltamivir in humans is unknown and likely to be strain-dependent.
Table 6: Neuraminidase Amino Acid Substitutions Associated with Reduced Susceptibility to Oseltamivir
*All numbering is N2, except where indicated.Amino Acid Substitution*
Influenza A N1 (N1 numbering in brackets)
I117V (I117V), E119V (E119V), R152K (R152K), Y155H (Y155H), F173V (F174V), D198G/N (D199G/N), I222K/R/T/V (I223K/R/T/V), S246G/N (S247G/N), G248R+I266V (G249R+I267V), H274Y(H275Y), N294S (N295S), Q312R+I427T (Q313R+I427T), N325K (N325K), R371K (R368K)
Influenza A N2
E41G, E119I/V, D151V, I222L/V, Q226H, SASG245-248 deletion, S247P, R292K, N294S
Influenza B (B numbering in brackets)
E119A (E117A), P141S (P139S), G142R (G140R), R152K (R150K), D198E/N/Y (D197E/N/Y), I222L/T/V (I221L/T/V), A246D/S/T (A245D/S/T), H274Y (H273Y), N294S (N294S), R371K (R374K), G402S (G407S)
Selection of influenza A viruses resistant to oseltamivir can occur at higher frequencies in children. Oseltamivir treatment‐associated resistance in pediatric treatment studies has been detected at frequencies of 27% to 37% and 3% to 18% (3/11 to 7/19 and 1/34 to 9/50 post‐treatment isolates, respectively) for influenza A/H1N1 virus and influenza A/H3N2 virus, respectively.
In immunocompromised adults and pediatrics (1 year of age and older), selection of influenza viruses resistant to oseltamivir can occur at higher frequencies than in the otherwise healthy population. In a treatment study of immunocompromised subjects, treatment‐associated genotypic resistance was detected in 27% (8/30), 12% (6/52), and 0% (0/42) of influenza A/H1N1, A/H3N2, and B virus infections, respectively. Treatment‐emergent resistance was observed at a higher frequency in hematopoietic stem cell transplant recipients (32%; 6/19).
The frequency of resistance selection to oseltamivir and the prevalence of such resistant virus vary seasonally and geographically.
Circulating seasonal influenza strains expressing neuraminidase resistance-associated substitutions have been observed in individuals who have not received oseltamivir treatment. The oseltamivir resistance-associated substitution H275Y was found in more than 99% of US-circulating 2008 H1N1 influenza virus isolates. The 2009 H1N1 influenza virus ("swine flu") was almost uniformly susceptible to oseltamivir; however, the frequency of circulating resistant variants can change from season to season. Prescribers should consider available information from the CDC on influenza virus drug susceptibility patterns and treatment effects when deciding whether to use oseltamivir phosphate for oral suspension.
Cross-resistance
Cross-resistance between oseltamivir and zanamivir has been observed in neuraminidase biochemical assays. The H275Y (N1 numbering) or N294S (N2 numbering) oseltamivir resistance-associated substitutions observed in the N1 neuraminidase subtype, and the E119V or N294S oseltamivir resistance-associated substitutions observed in the N2 subtype (N2 numbering), are associated with reduced susceptibility to oseltamivir but not zanamivir. The Q136K and K150T zanamivir resistance-associated substitutions observed in N1 neuraminidase, or the S250G zanamivir resistance-associated substitutions observed in influenza B virus neuraminidase, confer reduced susceptibility to zanamivir but not oseltamivir. The R292K oseltamivir resistance-associated substitution observed in N2, and the I222T, D198E/N, R371K, or G402S oseltamivir resistance-associated substitutions observed in influenza B virus neuraminidase, confer reduced susceptibility to both oseltamivir and zanamivir. These examples do not represent an exhaustive list of cross resistance-associated substitutions and prescribers should consider available information from the CDC on influenza drug susceptibility patterns and treatment effects when deciding whether to use oseltamivir phosphate for oral suspension.
No single amino acid substitution has been identified that could confer cross-resistance between the neuraminidase inhibitor class (oseltamivir, zanamivir) and the M2 ion channel inhibitor class (amantadine, rimantadine). However, a virus may carry a neuraminidase inhibitor associated substitution in neuraminidase and an M2 ion channel inhibitor-associated substitution in M2 and may therefore be resistant to both classes of inhibitors. The clinical relevance of phenotypic cross-resistance evaluations has not been established.
Immune Response
No influenza vaccine/oseltamivir interaction study has been conducted. In studies of naturally acquired and experimental influenza, treatment with oseltamivir phosphate for oral suspension did not impair normal humoral antibody response to infection.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In 2-year carcinogenicity studies in mice and rats given daily oral doses of the prodrug oseltamivir phosphate up to 400 mg/kg and 500 mg/kg, respectively, the prodrug and the active form oseltamivir carboxylate induced no statistically significant increases in tumors over controls. The mean maximum daily exposures to the prodrug in mice and rats were approximately 130- and 320-fold, respectively, greater than those in humans at the recommended clinical dose based on AUC comparisons. The respective safety margins of the exposures to the active oseltamivir carboxylate were 15- and 50-fold.
Oseltamivir was found to be non-mutagenic in the Ames test and the human lymphocyte chromosome assay with and without enzymatic activation and negative in the mouse micronucleus test. It was found to be positive in a Syrian Hamster Embryo (SHE) cell transformation test. Oseltamivir carboxylate was non-mutagenic in the Ames test and the L5178Y mouse lymphoma assay with and without enzymatic activation and negative in the SHE cell transformation test.
In a fertility and early embryonic development study in rats, doses of oseltamivir at 50 mg/kg/day, 250 mg/kg/day, and 1500 mg/kg/day were administered to females for 2 weeks before mating, during mating and until day 6 of pregnancy. Males were dosed for 4 weeks before mating, during mating, and for 2 weeks after mating. There were no effects on fertility, mating performance or early embryonic development at any dose level. The highest dose in this study was approximately 115 times the human systemic exposure (AUC0-24h) of oseltamivir carboxylate that occurs after administration of the maximum recommended human dose.
-
14 CLINICAL STUDIES
14.1 Treatment of Influenza
Adults
Two randomized, placebo-controlled, double-blind clinical trials of oseltamivir phosphate were conducted in adults between 18 and 65 years old, one in the U.S. and one outside the U.S., for the treatment of acute uncomplicated influenza. Eligible subjects had fever of at least 100ºF, accompanied by at least one respiratory symptom (cough, nasal symptoms, or sore throat) and at least one systemic symptom (myalgia, chills/sweats, malaise, fatigue, or headache), and influenza virus was known to be circulating in the community. Subjects were randomized to receive oral oseltamivir phosphate suspension or placebo for 5 days. All enrolled subjects were allowed to take fever-reducing medications.
Of 1,355 subjects enrolled in these two trials, 849 (63%) subjects were influenza-infected (median age 34 years; 52% male; 90% Caucasian; 31% smokers). Of the 849 influenza-infected subjects, 95% were infected with influenza A, 3% with influenza B, and 2% with influenza of unknown type.
Study medication was started within 40 hours of onset of symptoms and administered twice daily for 5 days. Subjects were required to self-assess the influenza-associated symptoms (nasal congestion, sore throat, cough, aches, fatigue, headaches, and chills/sweats) twice daily as "none," "mild," "moderate," or "severe". Time to improvement was calculated from the time of treatment initiation to the time when all symptoms were assessed as "none" or "mild". In both trials, there was a 1.3-day reduction in the median time to improvement in influenza-infected subjects who received oseltamivir phosphate 75 mg twice a day for 5 days compared to subjects who received placebo. Subgroup analyses by gender showed no differences in the treatment effect of oseltamivir phosphate in men and women.
In the treatment of influenza, no increased efficacy was demonstrated in subjects who received higher doses of oseltamivir phosphate for oral suspension.
Adolescents and Adults with Chronic Cardiac or Respiratory Disease
A double-blind, placebo-controlled, multicenter trial was unable to demonstrate efficacy of oseltamivir phosphate (75 mg twice daily for 5 days) in the treatment of influenza in adult and adolescent subjects (13 years or older) with chronic cardiac (excluding chronic idiopathic hypertension) or respiratory diseases, as measured by time to alleviation of all symptoms. However, in patients treated with oseltamivir phosphate there was a more rapid cessation of febrile illness. No difference in the incidence of influenza complications was observed between the treatment and placebo groups in this population.
Geriatric Subjects
Three double-blind placebo-controlled treatment trials were conducted in subjects who were at least 65 years of age in three consecutive seasons. The enrollment criteria were similar to that of adult trials with the exception of fever being defined as higher than 97.5°F. Of 741 subjects enrolled, 476 (65%) subjects were influenza-infected; of these, 95% were infected with influenza type A and 5% with influenza type B.
In the pooled analysis, there was a 1-day reduction in the median time to improvement in influenza-infected subjects who received oseltamivir phosphate for oral suspension 75 mg twice daily for 5 days compared to those who received placebo (p=NS) [see Use in Specific Populations ()]. Some seasonal variability was noted in the clinical efficacy outcomes.
Pediatric Subjects (1 year to 12 years of age)
One double-blind placebo-controlled treatment trial was conducted in pediatric subjects aged 1 year to 12 years (median age 5 years) who had fever (at least 100ºF) plus one respiratory symptom (cough or coryza) when influenza virus was known to be circulating in the community. Of 698 subjects enrolled in this trial, 452 (65%) were influenza-infected (50% male; 68% Caucasian). Of the 452 influenza-infected subjects, 67% were infected with influenza A and 33% with influenza B.
Efficacy in this trial was determined by the time to alleviation or resolution of influenza signs and symptoms, measured by a composite endpoint that required the following four individual conditions be met: i) alleviation of cough, ii) alleviation of coryza, iii) resolution of fever, and iv) parental opinion of a return to normal health and activity. Oseltamivir phosphate treatment of 2 mg per kg twice daily, started within 48 hours of onset of symptoms, reduced the total composite time to freedom from illness by 1.5 days compared to placebo. Subgroup analyses by gender showed no differences in the treatment effect of oseltamivir phosphate in male and female pediatric subjects.
Pediatric Subjects (2 weeks to less than 1 year of age)
Two open-label trials evaluated the safety and pharmacokinetics of oseltamivir and oseltamivir carboxylate in influenza-infected pediatric subjects 2 weeks to less than 1 year of age (including premature infants at least 36 weeks post conceptional age). Subjects received oseltamivir phosphate at doses ranging from 2 mg to 3.5 mg per kg twice daily for 5 days depending on subject age. These clinical trials were not designed to evaluate clinical efficacy or virologic response.
Of the 136 subjects under the age of 1 year enrolled and dosed in the trials, the majority of the subjects were male (55%), white (79%), non-Hispanic (74%), full term (76%) and infected with influenza A (80%). Pharmacokinetic data indicated that a dose of 3 mg per kg twice daily in pediatric subjects 2 weeks to less than 1 year of age provided oseltamivir phosphate concentrations similar to or higher than those observed in older pediatric subjects and adults receiving the approved dose and provided the basis for approval [see Adverse Reactions () and Use in Specific Populations (8.4)].
14.2 Prophylaxis of Influenza
Adult and Adolescent Subjects (13 years of age and older)
The efficacy of oseltamivir phosphate in preventing naturally occurring influenza illness has been demonstrated in three seasonal prophylaxis (community outbreak) clinical trials and one post-exposure prophylaxis trial in household contacts. The efficacy endpoint for all of these trials was the incidence of laboratory-confirmed clinical influenza defined as meeting all the following criteria (all signs and symptoms must have been recorded within 24 hours):
- oral temperature greater than or equal to 99.0ºF (37.2ºC),
- at least one respiratory symptom (cough, sore throat, nasal congestion),
- at least one constitutional symptom (aches and pain, fatigue, headache, chills/sweats), and
- either a positive virus isolation or a four-fold increase in virus antibody titers from baseline.
In a pooled analysis of two seasonal prophylaxis trials in healthy unvaccinated adults (aged 18 to 65 years), oseltamivir phosphate 75 mg once daily taken for 42 days during a community outbreak reduced the incidence of laboratory-confirmed clinical influenza from 5% (25/519) for the placebo group to 1% (6/520) for the oseltamivir phosphate group.
In the seasonal (community outbreak) prophylaxis trial in elderly residents of skilled nursing homes, about 80%, 43%, and 14% of the subjects were vaccinated, had cardiac disorders, and had chronic airway obstructive disorders, respectively. In this trial, subjects were randomized to oseltamivir phosphate 75 mg once daily or placebo taken orally for 42 days. The incidence of laboratory-confirmed clinical influenza was 4% (12/272) in the placebo-treated subjects compared to less than 1% (1/276) in the oseltamivir phosphate-treated subjects.
In the post-exposure prophylaxis trial in household contacts (aged 13 years or older) of an index influenza case, oseltamivir phosphate 75 mg once daily or placebo taken orally was administered within 48 hours of onset of symptoms in the index case and continued for 7 days (index cases did not receive oseltamivir phosphate treatment). The incidence of laboratory-confirmed clinical influenza was 12% (24/200) in the placebo-treated subjects compared to 1% (2/205) in the oseltamivir phosphate-treated subjects.
Pediatric Subjects (1 year to 12 years of age)
The efficacy of oseltamivir phosphate in preventing naturally occurring influenza illness was demonstrated in a randomized, open-label post-exposure prophylaxis trial in household contacts that included pediatric subjects aged 1 year to 12 years, both as index cases and as family contacts. All index cases in this trial received oseltamivir phosphate for oral suspension 30 mg to 60 mg taken orally once daily for 10 days. The efficacy parameter was the incidence of laboratory-confirmed clinical influenza in the household. Laboratory-confirmed clinical influenza was defined as meeting all of the following criteria:
- oral temperature at least 100°F (37.8°C),
- cough and/or coryza recorded within 48 hours, and
- either a positive virus isolation or a four-fold or greater increase in virus antibody titers from baseline or at illness visits.
Among household contacts 1 year to 12 years of age not already shedding virus at baseline, the incidence of laboratory-confirmed clinical influenza was lower in the group who received oseltamivir phosphate prophylaxis [3% (3/95)] compared to the group who did not receive oseltamivir phosphate prophylaxis [17% (18/106)].
Immunocompromised Subjects
A double-blind, placebo-controlled trial was conducted for seasonal prophylaxis of influenza in 475 immunocompromised subjects (including 18 pediatric subjects 1 year to 12 years of age) who had received solid organ (n=388; liver, kidney, liver and kidney) or hematopoietic stem cell transplants (n=87). Median time since transplant for solid organ transplant recipients was 1,105 days for the placebo group and 1,379 days for the oseltamivir phosphate group. Median time since transplant for hematopoietic stem cell transplant recipients was 424 days for the placebo group and 367 days for the oseltamivir phosphate group. Approximately 40% of subjects received influenza vaccine prior to entering the study. The primary efficacy endpoint was the incidence of confirmed clinical influenza, defined as oral temperature higher than 99.0°F (37.2°C) plus cough and/or coryza, all recorded within 24 hours, plus either a positive virus culture or a four-fold increase in virus antibody titers from baseline. Subjects received treatment with oseltamivir phosphate 75 mg or placebo once daily by mouth for 12 weeks. The incidence of confirmed clinical influenza was 3% (7/238) in the placebo group compared with 2% (5/237) in the oseltamivir phosphate group; this difference was not statistically significant. A secondary analysis was performed using the same clinical symptoms and RT-PCR for laboratory confirmation of influenza infection. Among subjects who were not already shedding virus at baseline, the incidence of RT-PCR-confirmed clinical influenza infection was 3% (7/231) in the placebo group and less than 1% (1/232) in the oseltamivir phosphate group.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Oseltamivir Phosphate for Oral Suspension (Supplied as Powder)
Supplied as a white to yellow powder blend in a glass bottle with a child-resistant closure, enclosed in a gusset pouch individually packed in a carton, NDC: 68788-8123-6.
After constitution, the powder blend produces a white to yellow tutti-frutti–flavored oral suspension. After constitution with 55 mL of water, each bottle delivers a usable volume of 60 mL of oral suspension equivalent to 360 mg oseltamivir base (6 mg/mL) [see Dosage and Administration ()].
Pharmacist: Discard the gusset pouch after reconstitution.
Storage
Store dry powder at 25ºC (77ºF); excursions permitted to 15º to 30ºC (59º to 86ºF) [See USP Controlled Room Temperature].
Store constituted oral suspension under refrigeration for up to 17 days at 2º to 8ºC (36º to 46ºF). Do not freeze. Alternatively, store constituted oral suspension for up to 10 days at 25ºC (77ºF); excursions permitted to 15º to 30ºC (59º to 86ºF) [See USP Controlled Room Temperature].
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Serious Skin/Hypersensitivity Reactions
Advise patients and/or caregivers of the risk of severe allergic reactions (including anaphylaxis) or serious skin reactions. Instruct patients and/or caregiver to stop oseltamivir phosphate for oral suspension and seek immediate medical attention if an allergic-like reaction occurs or is suspected [see Warnings and Precautions ()].
Neuropsychiatric Events
Advise patients and/or caregivers of the risk of neuropsychiatric events in oseltamivir phosphate for oral suspension-treated patients with influenza and instruct patients to contact their physician if they experience signs of abnormal behavior while receiving oseltamivir phosphate for oral suspension [see Warnings and Precautions ()].
Important Dosing Information
Instruct patients to begin treatment with oseltamivir phosphate for oral suspension as soon as possible from the first appearance of flu symptoms, within 48 hours of onset of symptoms. Similarly, instruct patients to start taking oseltamivir phosphate for oral suspension for prevention as soon as possible after exposure [see Dosage and Administration (2)]. Instruct patients to take any missed doses as soon as they remember, except if it is near the next scheduled dose (within 2 hours), and then continue to take oseltamivir phosphate for oral suspension at the usual times.
Influenza Vaccines
Instruct patients that oseltamivir phosphate for oral suspension is not a substitute for receiving an annual flu vaccination. Patients should continue receiving an annual flu vaccination according to guidelines on immunization practices. Because of the potential for oseltamivir phosphate for oral suspension to inhibit replication of live attenuated influenza vaccine (LAIV) and possibly reduce efficacy of LAIV, avoid administration of LAIV within 2 weeks or 48 hours after oseltamivir phosphate for oral suspension administration, unless medically necessary [see Drug Interactions ()].
Fructose Intolerance
Inform patients with hereditary fructose intolerance that one dose of 75 mg Oseltamivir Phosphate for Oral Suspension (supplied as powder) delivers 2 grams of sorbitol. Inform patients with hereditary fructose intolerance that this is above the daily maximum limit of sorbitol and may cause dyspepsia and diarrhea [see Warnings and Precautions ()].
Marketed by:
Ajanta Pharma USA Inc.
Bridgewater, NJ 08807.
Made in INDIA
#All trademarks are the properties of their respective owners
Revised: 05/2023Relabeled By: Preferred Pharmaceuticals Inc.
-
PATIENT PACKAGE INSERT
PATIENT INFORMATION
Oseltamivir Phosphate (oh'' sel tam' i vir fos' fate)
for oral suspensionWhat is Oseltamivir Phosphate for Oral Suspension?
Oseltamivir phosphate for oral suspension is a prescription medicine used to:- treat the flu (influenza) in people 2 weeks of age and older who have had flu symptoms for no more than two days.
- prevent the flu in people who are 1 year of age and older.
It is not known if oseltamivir phosphate for oral suspension is:
- effective in people who start treatment after 2 days of developing flu symptoms.
- effective for the treatment of the flu in people with long-time (chronic) heart problems or breathing problems.
- effective for the treatment or prevention of flu in people who have weakened immune systems (immunocompromised)
- safe and effective for the treatment of the flu in children less than 2 weeks of age.
- safe and effective in the prevention of the flu in children less than 1 year of age.
Oseltamivir phosphate for oral suspension does not treat or prevent illness that is caused by infections other than the influenza virus.
Oseltamivir phosphate for oral suspension does not prevent bacterial infections that may happen with the flu.
Oseltamivir phosphate for oral suspension is not recommended for people with end-stage renal disease (ESRD) who are not receiving dialysis.Oseltamivir phosphate for oral suspension does not take the place of receiving a flu vaccination. Talk to your healthcare provider about when you should receive an annual flu vaccination.
Who should not take Oseltamivir phosphate for oral suspension?
Do not take oseltamivir phosphate for oral suspension if you are allergic to oseltamivir phosphate or any of the ingredients in Oseltamivir phosphate for oral suspension. See the end of this leaflet for a complete list of ingredients in oseltamivir phosphate for oral suspension.What should I tell my healthcare provider before taking oseltamivir phosphate for oral suspension?
Before you take oseltamivir phosphate for oral suspension, tell your healthcare provider if you:- have kidney problems
- have a history of fructose (fruit sugar) intolerance. Oseltamivir phosphate for oral suspension contains sorbitol and may cause stomach upset and diarrhea in people who are fructose intolerant.
- have any other medical conditions
- are pregnant or plan to become pregnant. Available information indicate that oseltamivir phosphate for oral suspension does not increase the risk of birth defects.
- are breastfeeding or plan to breastfeed. Oseltamivir phosphate can pass into breast milk in small amounts.
Tell your healthcare provider about all the medicines you take, including prescription or over-the-counter medicines, vitamins, and herbal supplements.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take oseltamivir phosphate for oral suspension?
- Take oseltamivir phosphate for oral suspension exactly as your healthcare provider tells you to.
- Take oseltamivir phosphate for oral suspension with food or without food. There is less chance of stomach upset if you take oseltamivir phosphate for oral suspension with food.
- If you miss a dose of oseltamivir phosphate for oral suspension, take it as soon as you remember. If it is 2 hours or less before your next dose, do not take the missed dose. Take your next dose of oseltamivir phosphate for oral suspension at your scheduled time. Do not take 2 doses at the same time.
If your healthcare provider or pharmacist has instructed you to take oseltamivir phosphate for oral suspension, read the detailed Instructions for Use at the end of this leaflet. Ask your pharmacist if you have any questions.
What are the possible side effects of oseltamivir phosphate for oral suspension?
Oseltamivir phosphate for oral suspension may cause serious side effects, including:-
Serious skin and allergic reactions. Oseltamivir phosphate for oral suspension can cause serious skin and allergic reactions. Stop taking
- o seltamivir phosphate for oral suspension and get medical help right away if you get any of the following symptoms:
- o skin rash or hives
- o your skin blisters and peels
- o blisters or sores in your mouth
- o itching
- o swelling of your face, eyes, lips, tongue, or throat
- o trouble breathing
- o chest pain or tightnes
- Change in behavior. People, especially children, who have the flu, can develop nervous system problems and abnormal behavior that can lead to death. During treatment with oseltamivir phosphate for oral suspension, tell your healthcare provider right away if you or your child have confusion, speech problems, shaky movements, seizures, or start hearing voices or seeing things that are not really there (hallucinations).
The most common side effects of oseltamivir phosphate for oral suspension when used for treatment of the flu include nausea, vomiting, and headache.
The most common side effect of oseltamivir phosphate for oral suspension when used for prevention of the flu include nausea, vomiting, headache, and pain.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of oseltamivir phosphate for oral suspension.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store oseltamivir phosphate for oral suspension?
- Store Oseltamivir phosphate for oral suspension in the refrigerator for up to 17 days between 36ºF to 46ºF (2ºC to 8ºC). Do not freeze.
- Store Oseltamivir phosphate for oral suspension for up to 10 days at room temperature between 68°F to 77°F (20°C to 25°C).
- Safely throw away any unused oseltamivir phosphate for oral suspension that is out of date or no longer needed.
Oseltamivir phosphate for oral suspension comes in a child-resistant package.
Keep oseltamivir phosphate for oral suspension and all medicines out of the reach of children.
General information about the safe and effective use of oseltamivir phosphate for oral suspension.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use oseltamivir phosphate for oral suspension for a condition for which it was not prescribed. Do not give oseltamivir phosphate for oral suspension to other people, even if they have the same symptoms you have. It may harm them. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about oseltamivir phosphate for oral suspension that is written for health professionals.What are the ingredients in oseltamivir phosphate for oral suspension?
Active ingredient: Oseltamivir Phosphate, USP
Inactive ingredients:
Oseltamivir phosphate for oral suspension: trisodium citrate, saccharin sodium, sodium benzoate, sorbitol, titanium dioxide, tutti-frutti flavoring, and xanthan gum.Marketed by:
Ajanta Pharma USA Inc.
Bridgewater, NJ 08807.Made in INDIA
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 05/2023
#All trademarks are the properties of their respective owners
-
PATIENT PACKAGE INSERT
INSTRUCTIONS FOR USE
Oseltamivir Phosphate (oh'' sel tam' i vir fos' fate)
for oral suspensionHow do I give a dose of Oseltamivir Phosphate for oral suspension?
Step 1. Shake the Oseltamivir Phosphate for oral suspension bottle well before each use.
Step 2. Open the bottle by pushing downward on the child resistant bottle cap and twisting it in the direction of the arrow.
Step 3. Measure the oral suspension with an appropriate oral dosing dispenser to be sure you get the correct dose. Contact your pharmacist if you do not have an appropriate oral dosing dispenser.
Step 4. Give the full contents of oral dosing dispenser directly into the mouth.
Step 5. Close the bottle with the child-resistant bottle cap after each use.
Step 6. Rinse oral dosing dispenser under running tap water and allow to air dry after each use.This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 05/2023
Relabeled By: Preferred Pharmaceuticals Inc.
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 68788-8123-6
Oseltamivir Phosphate for Oral Suspension
6 mg/mL
60 mL (usable volume after constitution)
Rx Only
ajanta
Relabeled By: Preferred Pharmaceuticals Inc. -
INGREDIENTS AND APPEARANCE
OSELTAMIVIR PHOSPHATE
oseltamivir phosphate for suspensionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68788-8123(NDC:27241-139) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OSELTAMIVIR PHOSPHATE (UNII: 4A3O49NGEZ) (OSELTAMIVIR ACID - UNII:K6106LV5Q8) OSELTAMIVIR ACID 6 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color WHITE (white to yellow) Score Shape Size Flavor TUTTI FRUTTI Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68788-8123-6 1 in 1 CARTON 01/10/2022 1 1 in 1 POUCH 1 60 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212784 01/10/2022 Labeler - Preferred Pharmaceuticals Inc. (791119022) Registrant - Preferred Pharmaceuticals Inc. (791119022) Establishment Name Address ID/FEI Business Operations Preferred Pharmaceuticals Inc. 791119022 RELABEL(68788-8123)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.