DERMFREE ORIGINAL NASAL by Jiangxi Hemei Pharmaceutical Co., Ltd 84010-011 COMPLETED
DERMFREE ORIGINAL NASAL by
Drug Labeling and Warnings
DERMFREE ORIGINAL NASAL by is a Otc medication manufactured, distributed, or labeled by Jiangxi Hemei Pharmaceutical Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
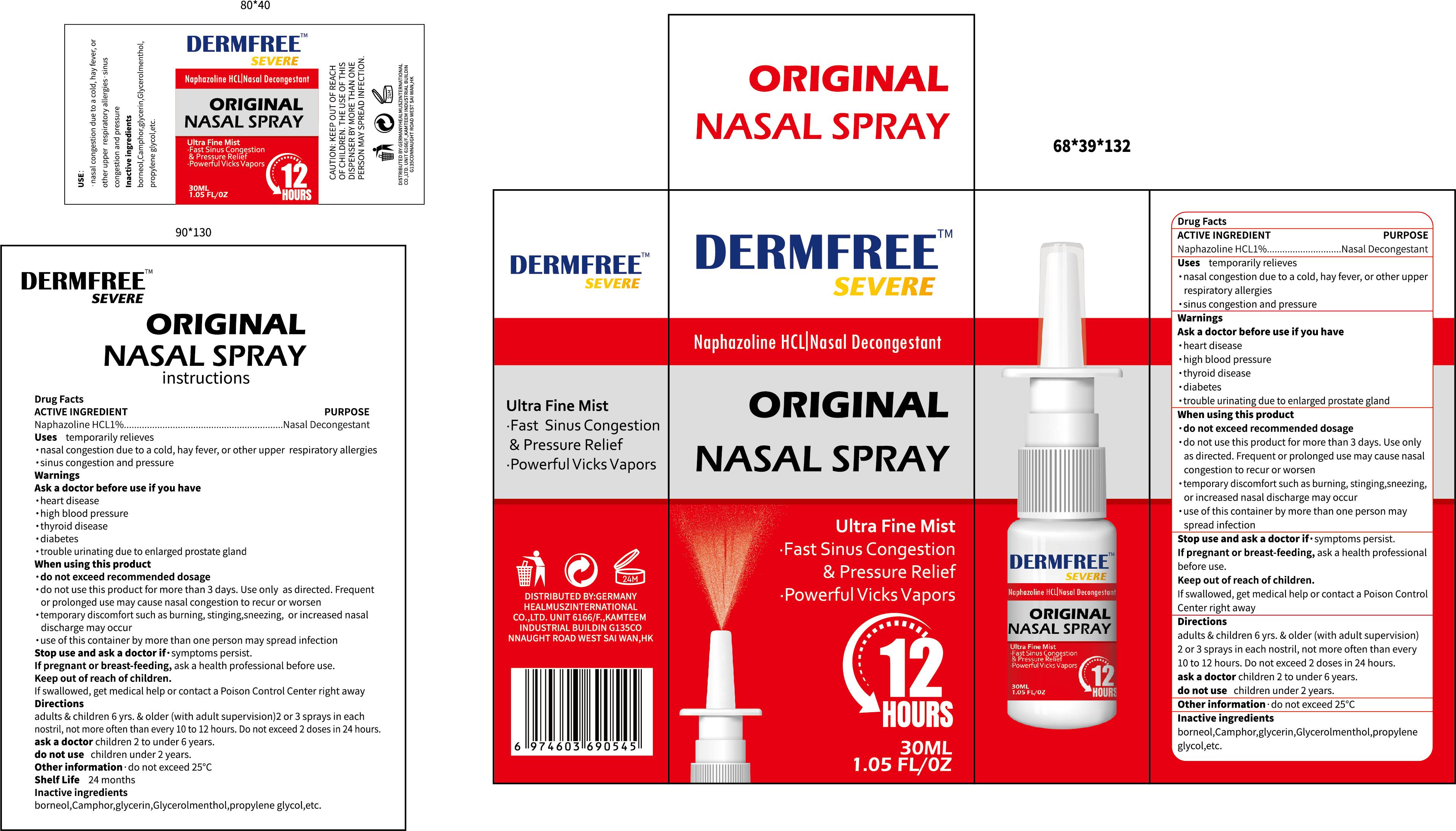
DERMFREE ORIGINAL NASAL- naphazoline hcl 1%original nasal spray
Jiangxi Hemei Pharmaceutical Co., Ltd
----------
84010-011 COMPLETED
Use
temporarily relieves*nasal congestion due to a cold, hay fever, or other upper respiratory allergies*sinus congestion and pressure
Warnings
Ask a doctor before use if you have
*heart disease
·high blood pressure
*thyroid disease
*diabetes
*trouble urinating due to enlarged prostate gland
When Using
*do not exceed recommended dosage
*do not use this product for more than 3 days. Use only as directed. Frequentor prolonged use may cause nasal congestion to recur or worsentemporary discomfort such as burning, stinging,sneezing, or increased nasaldischarge may occur
Keep Oot Of Reach Of Children
ifswallowed, get medical help or contact a Poison Control Center right away
| DERMFREE ORIGINAL NASAL
naphazoline hcl 1%original nasal spray |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - Jiangxi Hemei Pharmaceutical Co., Ltd (724892056) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Jiangxi Hemei Pharmaceutical Co., Ltd | 724892056 | manufacture(84010-011) | |