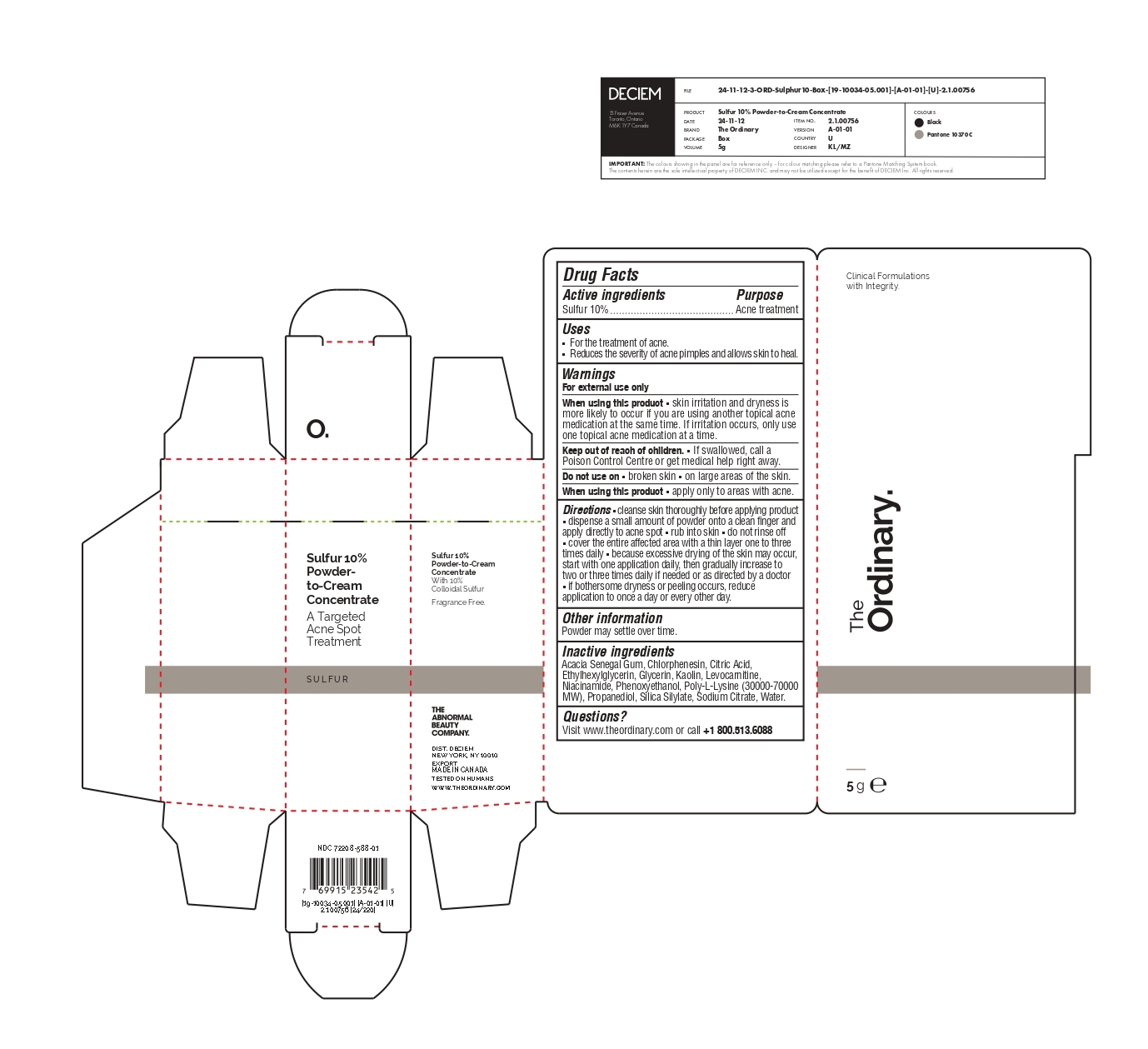
The Ordinary Sulfur 10% Powder-to-Cream Concentrate
The Ordinary Sulfur 10% Powder-to-Cream Concentrate by
Drug Labeling and Warnings
The Ordinary Sulfur 10% Powder-to-Cream Concentrate by is a Otc medication manufactured, distributed, or labeled by Deciem. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
THE ORDINARY SULFUR 10% POWDER-TO-CREAM CONCENTRATE- sulfur powder
Deciem
----------
The Ordinary Sulfur 10% Powder-to-Cream Concentrate
| THE ORDINARY SULFUR 10% POWDER-TO-CREAM CONCENTRATE
sulfur powder |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Deciem (203133665) |
Revised: 11/2025
Document Id: 43802ab6-a538-228f-e063-6394a90a4ffd
Set id: 1752bcf0-c1d1-838d-e063-6294a90a5d24
Version: 4
Effective Time: 20251113
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.