LASTACAFT- alcaftadine solution/ drops
LASTACAFT by
Drug Labeling and Warnings
LASTACAFT by is a Prescription medication manufactured, distributed, or labeled by Allergan, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LASTACAFT® safely and effectively. See full prescribing information for LASTACAFT®.
LASTACAFT® (alcaftadine ophthalmic solution)
For Topical Ophthalmic Use
Initial U.S. Approval: 2010RECENT MAJOR CHANGES
- Contraindications, Hypersensitivity (4) 12/2014
INDICATIONS AND USAGE
LASTACAFT® is an H1 histamine receptor antagonist indicated for the prevention of itching associated with allergic conjunctivitis. (1)
DOSAGE AND ADMINISTRATION
Instill one drop in each eye once daily. (2)
DOSAGE FORMS AND STRENGTHS
Ophthalmic solution containing alcaftadine, 0.25% (2.5 mg/mL) (3)
CONTRAINDICATIONS
Hypersensitivity. (4)
WARNINGS AND PRECAUTIONS
- To minimize the risk of eye injury and contamination, do not touch dropper tip to eyelids and surrounding areas, or any other surface. Keep bottle tightly closed when not in use. (5.1)
- LASTACAFT® should not be used to treat contact lens-related irritation. (5.2)
- Remove contact lenses prior to instillation of LASTACAFT®. (5.2)
ADVERSE REACTIONS
The most common ocular adverse reactions, occurring in less than 4% of eyes treated with LASTACAFT®, were eye irritation, burning and/or stinging on instillation, eye redness, and eye pruritus. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Allergan at 1-800-433-8871 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2015
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Potential for Eye Injury and Contamination
5.2 Contact Lens Use
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Non-ocular Adverse Reactions
6.3 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Potential for Eye Injury and Contamination
To minimize eye injury and contamination of the dropper tip and solution, care should be taken not to touch the eyelids or surrounding areas with the dropper tip of the bottle. Keep bottle tightly closed when not in use.
5.2 Contact Lens Use
Patients should be advised not to wear a contact lens if their eye is red.
LASTACAFT® should not be used to treat contact lens-related irritation.
LASTACAFT® should not be instilled while wearing contact lenses. Remove contact lenses prior to instillation of LASTACAFT®. The preservative in LASTACAFT®, benzalkonium chloride, may be absorbed by soft contact lenses. Lenses may be reinserted after 10 minutes following administration of LASTACAFT®.
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
6.1 Clinical Studies Experience
The most frequent ocular adverse reactions, occurring in less than 4% of eyes treated with LASTACAFT®, were eye irritation, burning and/or stinging upon instillation, eye redness and eye pruritus.
6.2 Non-ocular Adverse Reactions
The most frequent non-ocular adverse reactions, occurring in less than 3% of subjects with eyes treated with LASTACAFT®, were nasopharyngitis and headache. Some of these events were similar to the underlying disease being studied.
6.3 Postmarketing Experience
The following adverse reactions have been identified during postmarketing use of LASTACAFT® in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These reactions include eye discharge, eye swelling, erythema of eyelid, eyelid edema, lacrimation increased, vision blurred, hypersensitivity reactions including swelling of the face or allergic dermatitis, and somnolence.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category B. Reproduction studies performed in rats and rabbits revealed no evidence of impaired female reproduction or harm to the fetus due to alcaftadine. Oral doses in rats and rabbits of 20 and 80 mg/kg/day, respectively, produced plasma exposure levels approximately 200 and 9000 times the plasma exposure at the recommended human ocular dose. There are however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
8.3 Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when LASTACAFT® is administered to a nursing woman.
-
11 DESCRIPTION
LASTACAFT® is a sterile, topically administered H1 receptor antagonist containing alcaftadine for ophthalmic use.
Alcaftadine is a white to yellow powder with an empirical formula of C19H21N3O and a molecular weight of 307.39.
Contains:
Active: alcaftadine 0.25% (2.5 mg/mL)
Inactives: benzalkonium chloride 0.005% as a preservative; edetate disodium; sodium phosphate, monobasic; purified water; sodium chloride; sodium hydroxide and/or hydrochloric acid (to adjust pH)
Chemical Name: 6,11-dihydro-11-(1-methyl-4-piperidinylidene)-5H-imidazo[2,1-b] [3] benzazepine-3-carboxaldehyde
Structural Formula:
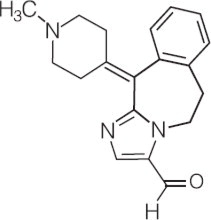
The drug product has a pH of approximately 7 and an osmolality of approximately 290 mOsm/kg.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Alcaftadine is an H1 histamine receptor antagonist and inhibitor of the release of histamine from mast cells. Decreased chemotaxis and inhibition of eosinophil activation has also been demonstrated.
12.3 Pharmacokinetics
Absorption
Following bilateral topical ocular administration of alcaftadine ophthalmic solution, 0.25%, the mean plasma Cmax of alcaftadine was approximately 60 pg/mL and the median Tmax occurred at 15 minutes. Plasma concentrations of alcaftadine were below the lower limit of quantification (10 pg/mL) by 3 hours after dosing. The mean Cmax of the active carboxylic acid metabolite was approximately 3 ng/mL and occurred at 1 hour after dosing. Plasma concentrations of the carboxylic acid metabolite were below the lower limit of quantification (100 pg/mL) by 12 hours after dosing. There was no indication of systemic accumulation or changes in plasma exposure of alcaftadine or the active metabolite following daily topical ocular administration.
Distribution
The protein binding of alcaftadine and the active metabolite are 39.2% and 62.7%, respectively.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Alcaftadine was not mutagenic or genotoxic in the Ames test, the mouse lymphoma assay or the mouse micronucleus assay.
Alcaftadine was found to have no effect on fertility of male and female rats at oral doses up to 20 mg/kg/day (approximately 200 times the plasma exposure at the recommended human ocular dose).
-
14 CLINICAL STUDIES
Clinical efficacy was evaluated in conjunctival allergen challenge (CAC) studies.
LASTACAFT® was more effective than its vehicle in preventing ocular itching in patients with allergic conjunctivitis induced by an ocular allergen challenge, both at 3 minutes post-dosing and at 16 hours post-dosing of LASTACAFT®.The safety of LASTACAFT® was evaluated in a randomized clinical study of 909 subjects over a period of 6 weeks.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
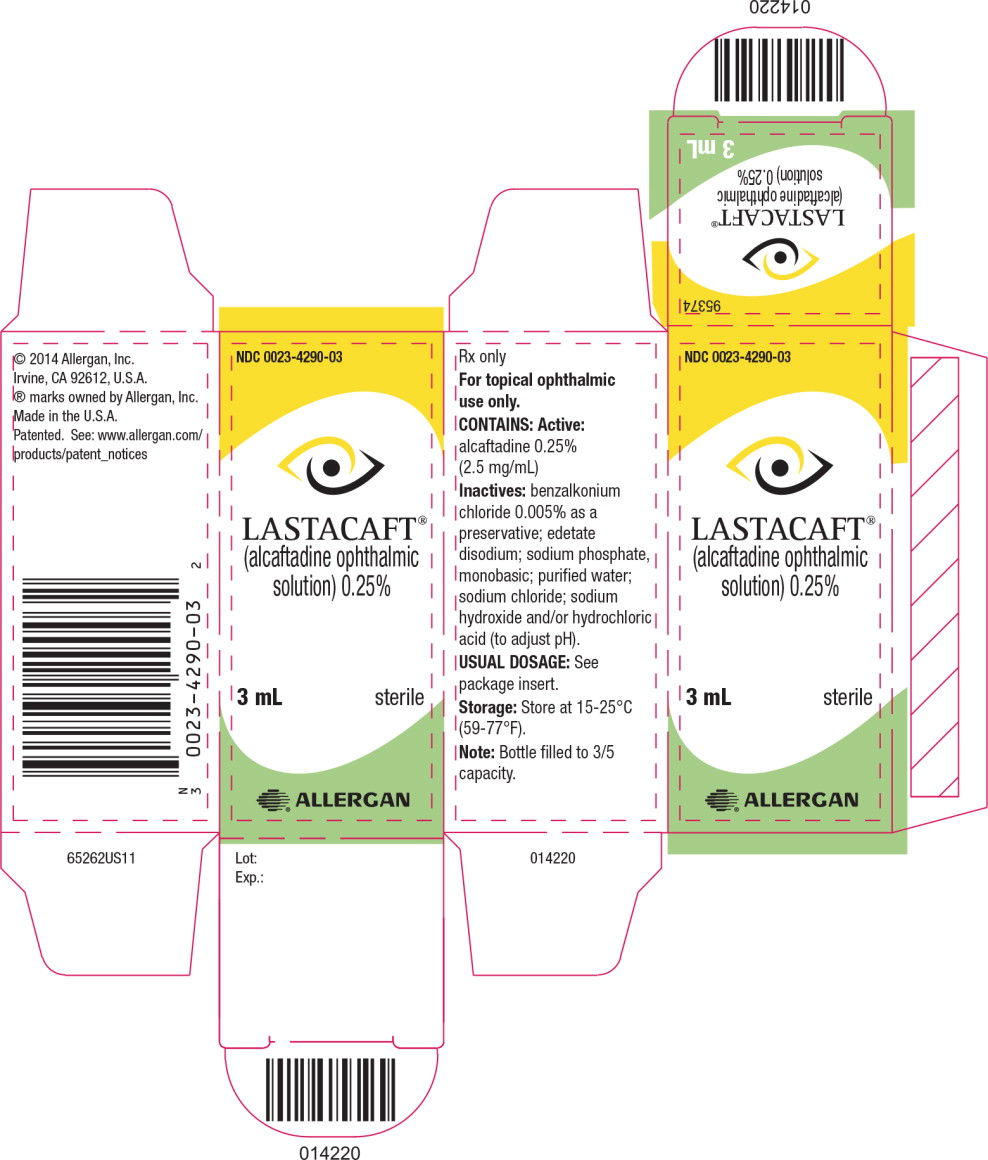
LASTACAFT® (alcaftadine ophthalmic solution) 0.25% is supplied in an opaque, white low-density polyethylene bottle with a white polystyrene cap.
3 mL fill in 5 mL bottle NDC: 0023-4290-03
-
17 PATIENT COUNSELING INFORMATION
Potential for Eye Injury and Sterility of Dropper Tip
To minimize eye injury and contamination of the dropper tip and solution, patients should be advised to not touch the eyelids or surrounding areas with the dropper tip, as this may contaminate the contents.
Concomitant Use with other Ophthalmic Products or Contact Lenses
If more than one topical ophthalmic drug is being used, the drugs should be administered at least five minutes apart.
Patients should be advised not to wear a contact lens if their eye is red. Patients should be advised that LASTACAFT® should not be used to treat contact lens-related irritation. Patients should also be advised to remove contact lenses prior to instillation of LASTACAFT®. The preservative in LASTACAFT®, benzalkonium chloride, may be absorbed by soft contact lenses. Lenses may be reinserted after 10 minutes following administration of LASTACAFT®.
© 2016 Allergan. All rights reserved.
Irvine, CA 92612, U.S.A.
All trademarks are the property of their respective owners.
Made in the U.S.A.
Patented. See: www.allergan.com/products/patent_notices
72468US13
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LASTACAFT
alcaftadine solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0023-4290 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength alcaftadine (UNII: 7Z8O94ECSX) (alcaftadine - UNII:7Z8O94ECSX) alcaftadine 2.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength benzalkonium chloride (UNII: F5UM2KM3W7) edetate disodium (UNII: 7FLD91C86K) sodium phosphate, monobasic (UNII: 3980JIH2SW) water (UNII: 059QF0KO0R) sodium chloride (UNII: 451W47IQ8X) sodium hydroxide (UNII: 55X04QC32I) hydrochloric acid (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0023-4290-01 1 in 1 CARTON 11/01/2010 1 1 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC: 0023-4290-03 1 in 1 CARTON 11/01/2010 2 3 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022134 11/01/2010 Labeler - Allergan, Inc. (144796497)
Trademark Results [LASTACAFT]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LASTACAFT 85168259 4071760 Live/Registered |
Allergan, Inc. 2010-11-03 |
 LASTACAFT 85088422 4003795 Live/Registered |
Allergan, Inc. 2010-07-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.