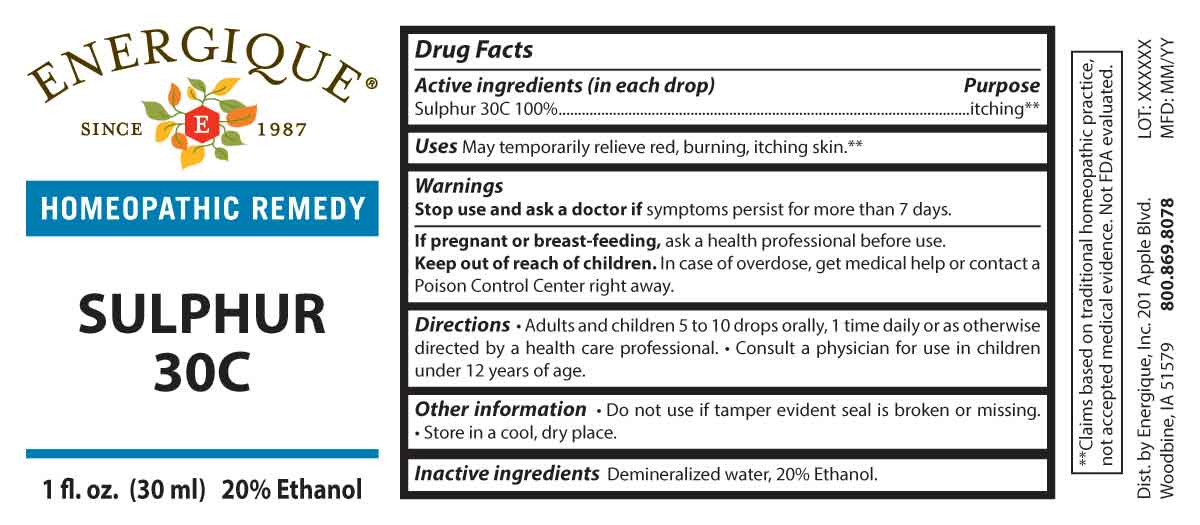
Sulphur by Energique, Inc. / Apotheca Company DRUG FACTS:
Sulphur by
Drug Labeling and Warnings
Sulphur by is a Homeopathic medication manufactured, distributed, or labeled by Energique, Inc., Apotheca Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SULPHUR 30C- sulphur liquid
Energique, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
DRUG FACTS:
PURPOSE:
Sulphur 30C - itching**
**Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
USES:
May temporarily relieve red, burning, itching skin.**
**Claims based on traditional homeopathic practice, not accepted medical evidence. Not FDA evaluated.
WARNINGS:
Stop use and ask a doctor if symptoms persist for more than 7 days.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if tamper evident seal is broken or missing. Store in a cool, dry place.
KEEP OUT OF REACH OF CHILDREN:
In case of overdose, get medical help or contact a Poison Control Center right away.
| SULPHUR
30C
sulphur liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Energique, Inc. (789886132) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(44911-0700) , api manufacture(44911-0700) , label(44911-0700) , pack(44911-0700) | |
Trademark Results [Sulphur]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SULPHUR 79280963 not registered Live/Pending |
H Sulphur Corp. 2020-01-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.