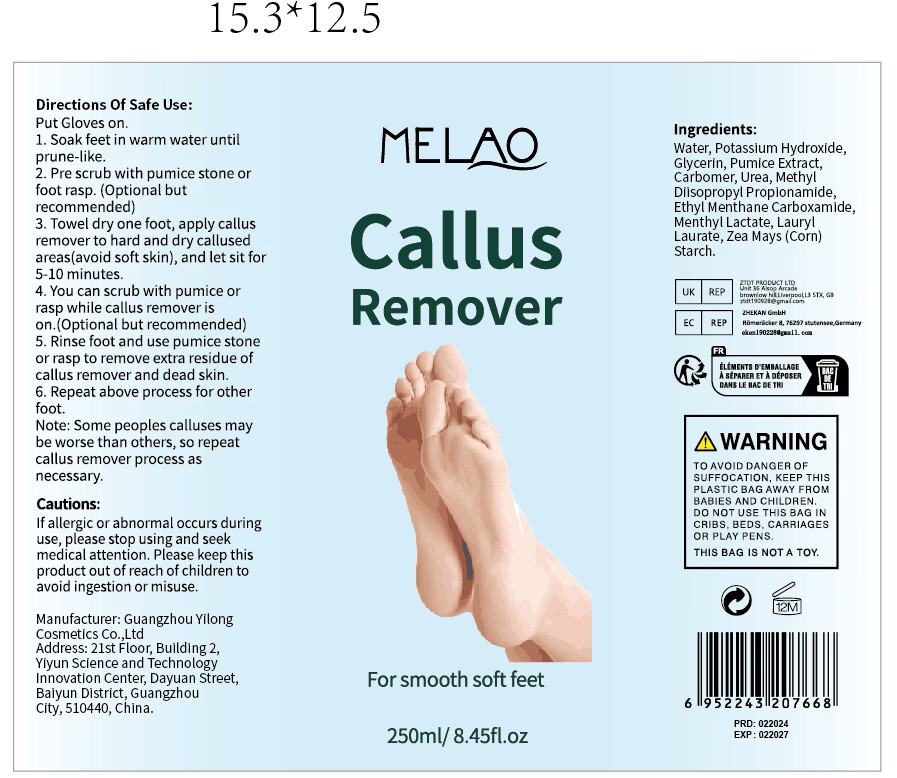
MELAO Callus remover by Guangzhou Yilong Cosmetics Co.,Ltd. MELAO Callus remover
MELAO Callus remover by
Drug Labeling and Warnings
MELAO Callus remover by is a Otc medication manufactured, distributed, or labeled by Guangzhou Yilong Cosmetics Co.,Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MELAO CALLUS REMOVER- pumice,urea gel
Guangzhou Yilong Cosmetics Co.,Ltd.
----------
MELAO Callus remover
Uses
Put Gloves on.
1. Soak feet in warm water until prune-like.
2. Pre scrub with pumice stone or foot rasp.(Optional but recommended)
3. Towel dry one foot, apply callus remover to hard and dry callused areas(avoid soft skin), and let sit for 5-10 minutes.
4. You can scrub with pumice or rasp while callus remover is on.(Optional but recommended)
5. Rinse foot and use pumice stone or rasp to remove extra residue of callus remover and dead skin.
6. Repeat above process for other foot.Note: Some peoples calluses maybe worse than others, so repeat callus remover process as necessary.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
1. Soak feet in warm water untilprune-like.
2. Pre scrub with pumice stone orfoot rasp.
3. Towel dry one foot, apply callusremover to hard
and dry callusedareas, and let sit for 5-10 minutes.
| MELAO CALLUS REMOVER
pumice,urea gel |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Guangzhou Yilong Cosmetics Co.,Ltd. (712647107) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Guangzhou Yilong Cosmetics Co.,Ltd. | 712647107 | manufacture(83566-276) | |