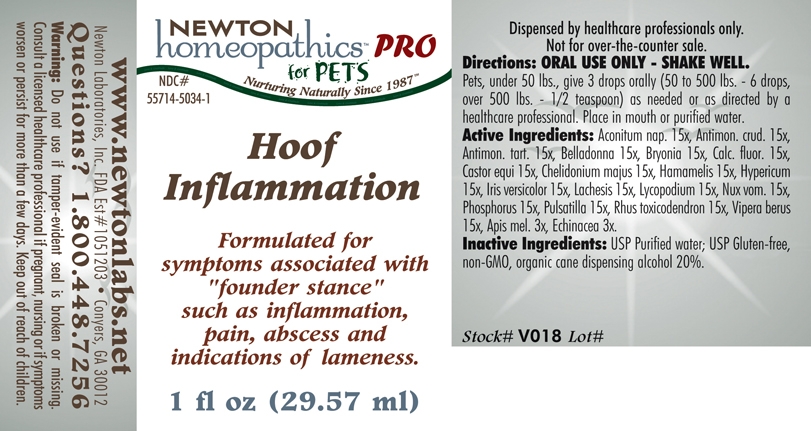
Hoof Inflammation by Newton Laboratories, Inc. Hoof Inflammation
Hoof Inflammation by
Drug Labeling and Warnings
Hoof Inflammation by is a Homeopathic medication manufactured, distributed, or labeled by Newton Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HOOF INFLAMMATION- aconitum nap., antimon. crud., antimon. tart., belladonna, bryonia, calc. fluor., castor equi, chelidonium majus, hamamelis, hypericum, iris versicolor, lachesis, lycopodium, nux vom., phosphorus, pulsatilla, rhus toxicodendron, vipera berus, apis mel., echinacea liquid
Newton Laboratories, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Hoof Inflammation
INDICATIONS & USAGE SECTION
Hoof Inflammation: Formulated for symptoms associated with "founder stance" such as inflammation, pain, abscess and indications of lameness.
DOSAGE & ADMINISTRATION SECTION
Directions: ORAL USE ONLY - SHAKE WELL. Pets, under 50 lbs., give 3 drops orally (50 to 500 lbs. - 6 drops, over 500 lbs. - 1/2 teaspoon) as needed or as directed by a healthcare professional. Place in mouth or purified water.
ACTIVE INGREDIENT SECTION
Aconitum nap. 15x, Antimon. crud. 15x, Antimon. tart. 15x, Belladonna 15x, Bryonia 15x, Calc. fluor. 15x., Castor equi 15x, Chelidonium majus 15x, Hamamelis 15x, Hypericum 15x, Iris versicolor 15x, Lachesis 15x, Lycopodium 15x, Nux vom. 15x, Phosphorus 15x, Pulsatilla 15x, Rhus toxicodendron 15x, Vipera berus 15x, Apis mel. 3x, Echinacea 3x.
INACTIVE INGREDIENT SECTION
Inactive Ingredients: USP Purified Water; USP Gluten-free, non-GMO, organic cane dispensing alcohol 20%.
QUESTIONS SECTION
www.newtonlabs.net Newton Laboratories, Inc. FDA Est # 1051203 - Conyers, GA 30012
Questions? 1.800.448.7256
WARNINGS SECTION
Warning: Do not use if tamper - evident seal is broken or missing. Consult a licensed healthcare professional if pregnant, nursing or if symptoms worsen or persist for more than a few days. Keep out of reach of children.
| HOOF INFLAMMATION
aconitum nap., antimon. crud., antimon. tart., belladonna, bryonia, calc. fluor., castor equi, chelidonium majus, hamamelis, hypericum, iris versicolor, lachesis, lycopodium, nux vom., phosphorus, pulsatilla, rhus toxicodendron, vipera berus, apis mel., echinacea liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Newton Laboratories, Inc. (788793610) |
| Registrant - Newton Laboratories, Inc. (788793610) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Newton Laboratories, Inc. | 788793610 | MANUFACTURE, API MANUFACTURE | |