A3519 NERVE BLOCK by Smiths Medical ASD, Inc. / Aplicare, Inc. / Kwang Myung Pharm. Co., Ltd.
A3519 NERVE BLOCK by
Drug Labeling and Warnings
A3519 NERVE BLOCK by is a Other medication manufactured, distributed, or labeled by Smiths Medical ASD, Inc., Aplicare, Inc., Kwang Myung Pharm. Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
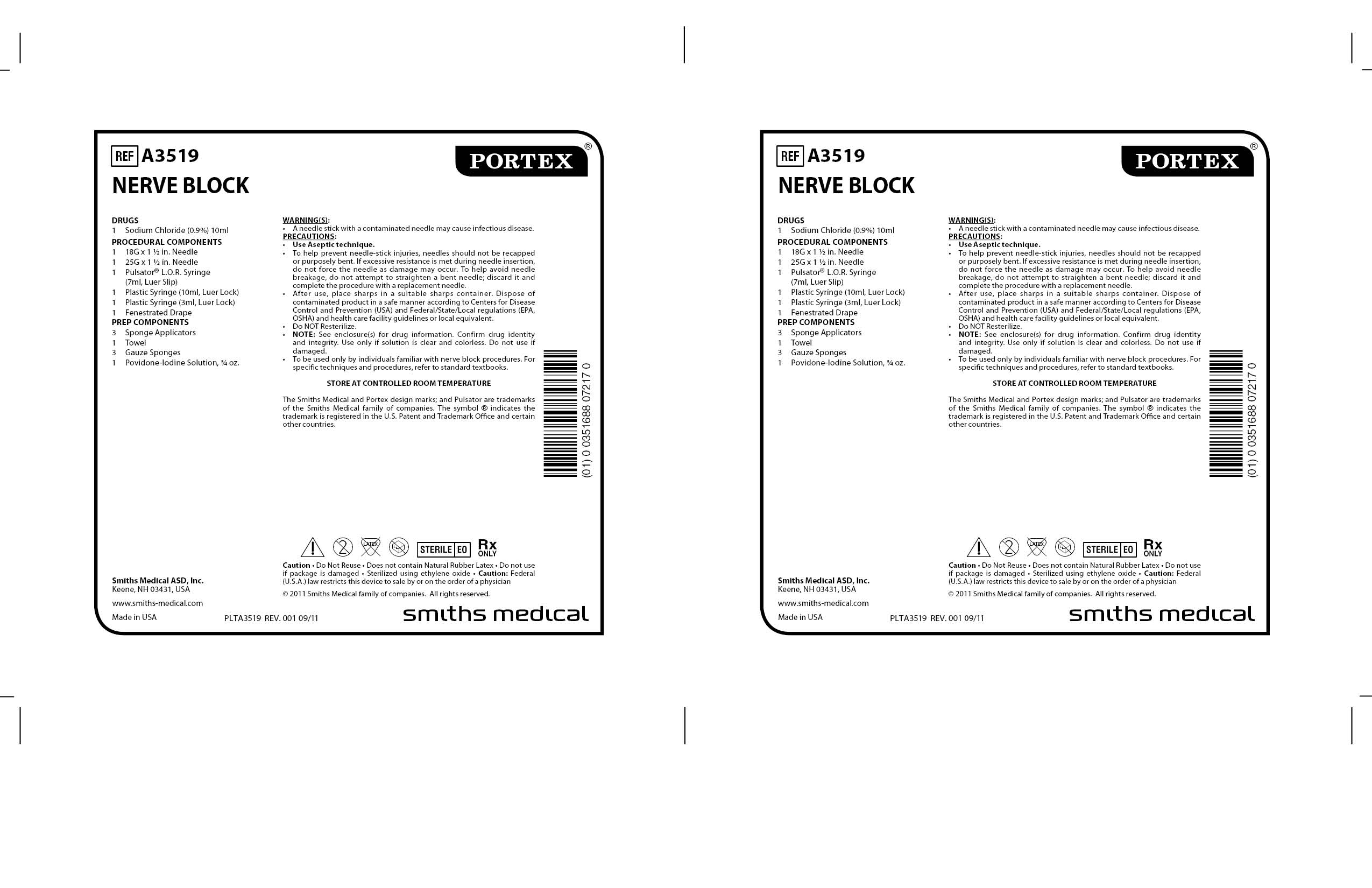
A3519 NERVE BLOCK- regional anesthesia kit
Smiths Medical ASD, Inc.
----------
<paragraph>
Spectra Medical Devices, Inc.
SODIUM CHLORIDE
INJECTION, USP, 0.9%
DESCRIPTIONSodium Chloride Injection, USP is a sterile, nonpyrogenic, isotonic solution of sodium chloride 0.9% (9mg/mL) in Water for Injection containing no antimicrobial agent or other added substance. The pH is between 4.5 and 7.0. Its chloride and sodium ion concentrates are approximately 0.154 mEq of each per milliliter and its calculated osmolality is 0.308 milliosmols per mL.
Sodium chloride occurs as colorless cubic crystals or white crystalline powder and has a saline taste. Sodium Chloride is freely soluble in water. It is soluble in glycerin and slightly soluble in alcohol. The empirical formula for sodium chloride is NaCl, and the molecular weight is 58.44.
CLINICAL PHARMACOLOGY
Sodium chloride comprises over 90% of the inorganic constituents of the blood serum. Sodium chloride in water dissociates to provide sodium (Na+) and (Cl-) ions. These ions are normal constituents of the body fluids (principally extracellular) and are essential for maintaining electrolyte balance. The small volume of fluid and amount of sodium chloride provided by Sodium Chloride Injection, USP, 0.9% when used only as a vehicle for parenteral injection of drugs, is unlikely to exert a significant effect on fluid and electrolyte balance except possibly in very small infants.
INDICATIONS AND USAGE
Sodium Chloride Injection is used to flush intravascular catheters or as a sterile, isontonic single dose vehicle, solvent, or diluent for substances to administered intravenously,k intramuscularly or sub-cutaneously and for other extemporaneously prepared single dose sterile solutions according to instructions of the manufacture of the drug to be administered.
WARNING
Sodium Chloride must be used with caution in the presence of congestive heart failure, circulatory insufficiency, kidney dysfunction or hypoproteinemia.
Excessive amounts of sodium chloride by any route may cause hypokalemia and acidosis. Excessive amounts by parental routes may precipitate congestive heart failure and acute pulmonary edema, especially seen in patients with preexisting cardiovascular disease and those receiving coricos-teroids, corticotrophin or other drugs that may give rise to sodium retention. For use in newborns, when a Sodium Chloride solution is required for preparation or diluting medications, or in flushing intravenous catheters, only preservative-free Sodium Chloride Injection, USP, 0.9% should be used.
PRECAUTIONS
GENERAL
Since Sodium Chloride Injection does not contain antimicrobial agents and is intended for single use, any unused amount must be discarded immediately following withdrawal of any portion of the contents of the vial or ampul. Do not open ampul until it is to be used.
Consult the manufactures instructions for choice of vehicle, appropriate dilution or volume for dissolving the drug to be injected, including the route and rate of injection.
PREGNANCY
CATEGORY C-Animal reproduction studies have not been conducted with Sodium Chloride Injection. It is also not known whether Sodium Chloride Injection can cause fetal harm when administered to a pregnant woman or can effect reproduction capacity. Sodium Chloride Injection should be given to a pregnant woman only if clearly needed.
<paragraph>
ADVERSE REACTIONSReactions which may occur because of this solution, added drugs or the technique of reconstitution or administration include febrile response, local tenderness, abscess, tissue necrosis or infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection and extravasations.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate countermeasures and if possible, retrieve and save the remainder of unused vehicle for examination.
OVERDOSAGE
When used as a diluent, solvent or intravascular flushing solution, this parental preparation is unlikely to pose a threat of sodium chloride or fluid overload except possible in very small infants. In the event these should occur, reevaluate the patient and institute appropriate corrective measures.
DOSAGE AND ADMINISTRATION
Before Sodium Chloride Injection, USP, 0.9% is used as a vehicle for the administration of a drug;specific references should be checked for any possible incompatibility with sodium chloride. The volume of the preparation to be used for diluting or dissolving any drug for injection is dependent on the vehicle concentration, dose and route of administration as recommended by the manufacture.
Sodium Chloride Injection, USP, 0.9% is also indicated for use in flushing intravenous catheters. Prior to and after administration of the medication, the intravenous catheter should be flushed in its entirety with Sodium Chloride Injection, USP, 0.9%. Use in accord with any warnings or precautions appropriate to the medication being administered as recommended by the manufacture. Parental drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
HOW SUPPLIED
5 mL ampuls packaged in box of 50 each (NDC-65282-1505-1)
10 mL ampuls packaged in box of 50 each (NDC-65282-1510-1)
30 mL ampuls packaged in box of 30 each (NDC-65282-1530-3)
STORAGE
Store at controlled room temperature 15-30 C (59-86 F). Avoid freezing.
Manufactured for:
Spectra Medical Devices, Inc. 260-F Fordham Road, Wilmington, MA 01887
By: KM. Pharm Co., LTD.
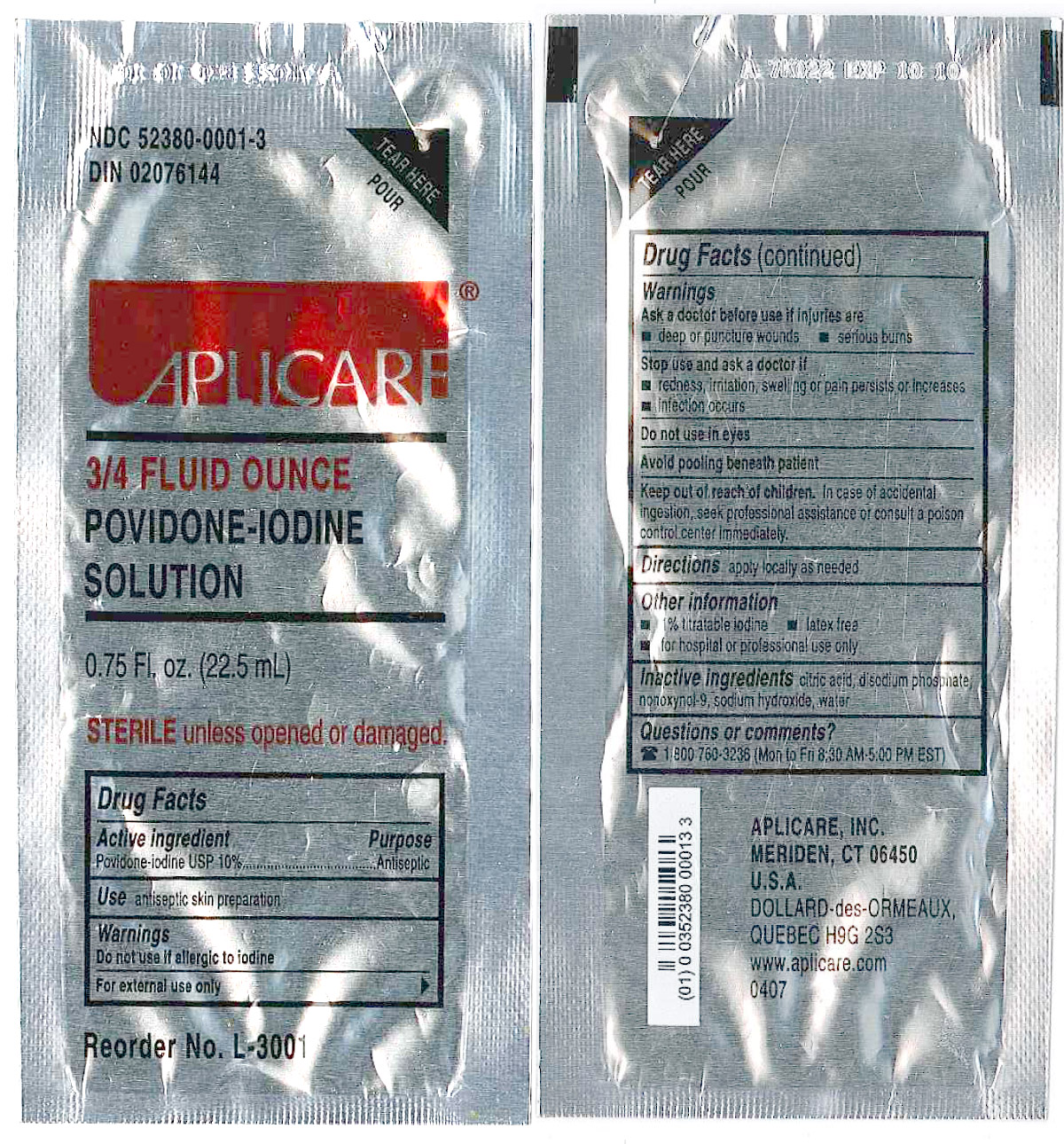
<paragraph>
3/4 Fluid Ounce Povidone Iodine
Povidone-iodine 10%
Antiseptic
Warnings
Do not use
- if allergic to iodine
- in the eyes
For external use only
Ask a doctor before use if injuries are
- deep or puncture wounds
- serious burns
Stop use and ask a doctor if
- redness, irritation, swelling or pain persists or increases
- infection occurs
Avoid pooling beneath patient
Keep out of reach of children.In case of accidental ingestion, seek professional assistance or consult a poison control center immediately.
| A3519 NERVE BLOCK
regional anesthesia kit kit |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Smiths Medical ASD, Inc. (137835299) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Smiths Medical ASD, Inc. | 137835299 | relabel, manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aplicare, Inc. | 081054904 | manufacture | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Spectra Medical Devices, Inc | 631099384 | manufacture | |