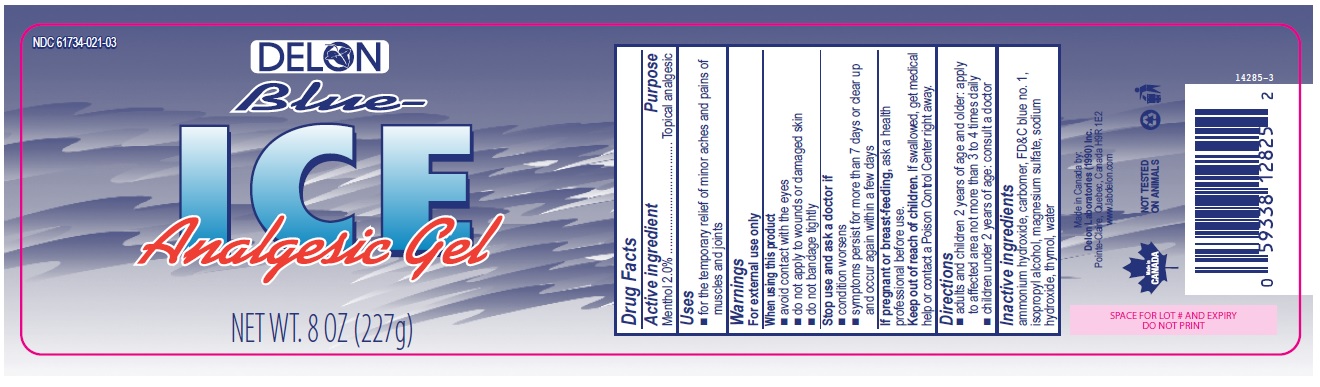
BLUE ICE ANALGESIC- menthol gel
Blue Ice Analgesic by
Drug Labeling and Warnings
Blue Ice Analgesic by is a Otc medication manufactured, distributed, or labeled by Delon Laboratories (1990) Ltd, Laboratoires Delon. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Inactive ingredients
- Delon 8oz (227g)
- Penetro 227g
-
INGREDIENTS AND APPEARANCE
BLUE ICE ANALGESIC
menthol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 61734-021 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 2 g in 100 g Inactive Ingredients Ingredient Name Strength THYMOL (UNII: 3J50XA376E) AMMONIA (UNII: 5138Q19F1X) SODIUM HYDROXIDE (UNII: 55X04QC32I) MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) CARBOMER 934 (UNII: Z135WT9208) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) WATER (UNII: 059QF0KO0R) ISOPROPYL ALCOHOL (UNII: ND2M416302) Product Characteristics Color blue Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61734-021-01 100 g in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/07/2010 11/06/2017 2 NDC: 61734-021-02 113.56 g in 1 CONTAINER; Type 0: Not a Combination Product 05/07/2010 12/04/2014 3 NDC: 61734-021-03 227 g in 1 CONTAINER; Type 0: Not a Combination Product 05/07/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 05/07/2010 Labeler - Delon Laboratories (1990) Ltd (248364184) Establishment Name Address ID/FEI Business Operations Delon Laboratories (1990) Inc. 243387722 manufacture(61734-021) , pack(61734-021) , label(61734-021)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.