BARRIERE- dimethicone cream
Barriere by
Drug Labeling and Warnings
Barriere by is a Otc medication manufactured, distributed, or labeled by WellSpring Pharmaceutical Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Purpose
- Use
- Warning
-
Directions
▪ For Skin Protection: Apply as needed
▪ For Treatment or Prevention of Diaper Rash: Change wet and soiled diapers promptly, cleanse the diaper area, and allow to dry. Apply cream liberally as often as necessary, with each diaper change, especially at bedtime or anytime when exposure to wet diapers may be prolonged.
- Other information
- Inactive ingredients
- Questions?
- Distributed by
- Package Label. Principal Display Panel
-
INGREDIENTS AND APPEARANCE
BARRIERE
dimethicone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 65197-426 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 0.2 g in 1 g Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) BUTYLPARABEN (UNII: 3QPI1U3FV8) CETYL ALCOHOL (UNII: 936JST6JCN) EDETATE SODIUM (UNII: MP1J8420LU) ETHYLPARABEN (UNII: 14255EXE39) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) METHYLPARABEN (UNII: A2I8C7HI9T) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) STEARIC ACID (UNII: 4ELV7Z65AP) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) Product Characteristics Color WHITE (white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65197-426-01 100 g in 1 TUBE; Type 0: Not a Combination Product 08/02/2010 12/31/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part347 08/02/2010 12/31/2020 Labeler - WellSpring Pharmaceutical Corporation (110999054)
Trademark Results [Barriere]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BARRIERE 86745332 5174095 Live/Registered |
GROUPE LUCIEN BARRIERE 2015-09-02 |
 BARRIERE 79110354 not registered Dead/Abandoned |
BPH Scagleitti A/S 2012-02-09 |
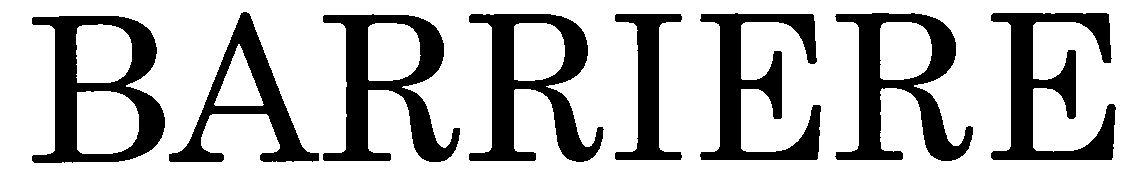 BARRIERE 79034342 3505438 Dead/Cancelled |
Mochigase Co., Ltd. 2007-01-11 |
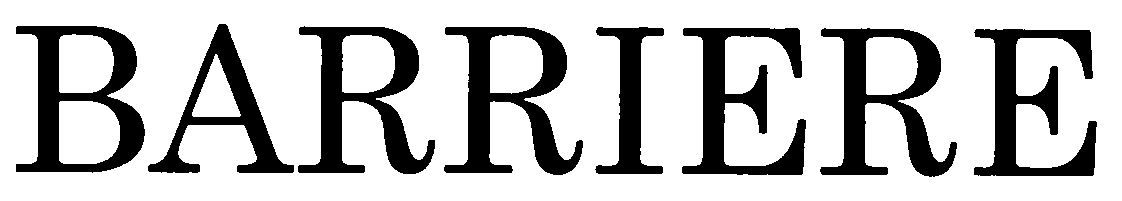 BARRIERE 79033001 3409343 Dead/Cancelled |
Mochigase Co., Ltd. 2006-11-13 |
 BARRIERE 77572985 3877422 Live/Registered |
Wellspring Pharmaceutical Corporation 2008-09-18 |
 BARRIERE 76016000 not registered Dead/Abandoned |
GT Laboratories, LLC 2000-04-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
