Ocean Potion Ice + Pain Relieving Soothing Gel
Ocean Potion by
Drug Labeling and Warnings
Ocean Potion by is a Otc medication manufactured, distributed, or labeled by Prime Enterprises. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
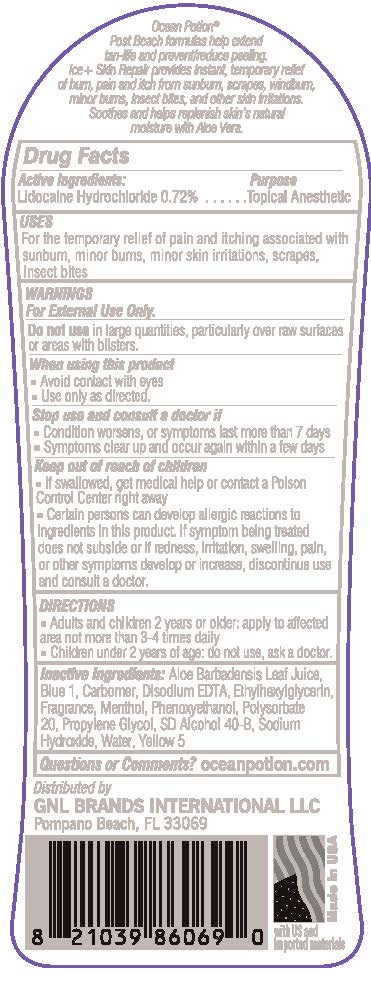
OCEAN POTION PAIN RELIEVING GEL- lidocaine hydrochloride gel
Prime Enterprises
----------
Ocean Potion Ice + Pain Relieving Soothing Gel
Uses
- for the temporary relief of pain and itching associated with sunburn, minor burns, minor skin irritations, scrapes, insect bites
Warnings
For exeternal use only
Stop use and consult a doctor if
- condition worsens, or symptoms last more than 7 days
- symptoms clear up and occur again within a few days
Keep out of reach of children
- if swallowed, get medical help or contact a Poison Control Center right away
- Certain persons can develop allergic reactions to ingredients in this product. If symptom being treated does not subside or if redness, irritation, swelling, pain, or other symptoms develop or increase, discontinue use and consult a doctor
Directions
- Adults and children 2 years or older: apply to affected area not more than 3 - 4 times daily
- Children under 2 years of age: do not use, ask a doctor
| OCEAN POTION
PAIN RELIEVING GEL
lidocaine hydrochloride gel |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Prime Enterprises (101946028) |
| Registrant - Prime Enterprises (101946028) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Prime Enterprises | 101946028 | pack(58443-0659) , analysis(58443-0659) , label(58443-0659) , manufacture(58443-0659) | |
Revised: 5/2024
Document Id: 18aa5557-0465-c658-e063-6394a90a555b
Set id: 18aa5557-0464-c658-e063-6394a90a555b
Version: 1
Effective Time: 20240517
Trademark Results [Ocean Potion]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 OCEAN POTION 88001823 5607272 Live/Registered |
SolSkyn Personal Care LLC 2018-06-15 |
 OCEAN POTION 87665604 not registered Dead/Abandoned |
GROWTH SCIENCE NUTRIENTS, INC. 2017-10-31 |
 OCEAN POTION 86166087 4596411 Live/Registered |
Sun & Skin Care Research, LLC 2014-01-15 |
 OCEAN POTION 85961665 4565412 Live/Registered |
Sun & Skin Care Research, LLC 2013-06-17 |
 OCEAN POTION 85820555 4424461 Live/Registered |
Prete, Michael Del 2013-01-10 |
 OCEAN POTION 78121749 2697922 Dead/Cancelled |
SUN AND SKIN CARE RESEARCH, INC. 2002-04-15 |
 OCEAN POTION 78121346 2865642 Live/Registered |
SUN & SKIN CARE RESEARCH, LLC 2002-04-12 |
 OCEAN POTION 78111309 2663419 Dead/Cancelled |
SUN & SKIN CARE RESEARCH, LLC 2002-02-27 |
 OCEAN POTION 78107715 2724181 Live/Registered |
SUN & SKIN CARE RESEARCH, LLC 2002-02-08 |
 OCEAN POTION 76159891 2766091 Live/Registered |
SUN & SKIN CARE RESEARCH, LLC 2000-11-03 |
 OCEAN POTION 76105836 not registered Dead/Abandoned |
John Frieda Professional Hair Care, Inc. 2000-08-09 |
 OCEAN POTION 75327410 not registered Dead/Abandoned |
Burt's Bees, Inc. 1997-07-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.