SENEXON- senna tablet, coated
SENEXON by
Drug Labeling and Warnings
SENEXON by is a Otc medication manufactured, distributed, or labeled by Aphena Pharma Solutions - Tennessee, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- ASK DOCTOR/PHARMACIST
- ASK DOCTOR
- WARNINGS
- STOP USE
- PREGNANCY OR BREAST FEEDING
- PURPOSE
- QUESTIONS
- KEEP OUT OF REACH OF CHILDREN
- INACTIVE INGREDIENT
-
DOSAGE & ADMINISTRATION
Dosage and Administration
Adults and children 12 years and over - 2 tablets once a day - maximum dosage - 4 tablets twice a day
Children 6 to under 12 years - 1 tablet once a day maximum dosage - 2 tablets twice a day
children 2 to under 6 years - 1/2 tablet once a day - maximum dosage - 1 tablet twice a day
- INDICATIONS & USAGE
-
Repackaging Information
Please reference the How Supplied section listed above for a description of individual tablets. This drug product has been received by Aphena Pharma - TN in a manufacturer or distributor packaged configuration and repackaged in full compliance with all applicable cGMP regulations. The package configurations available from Aphena are listed below:
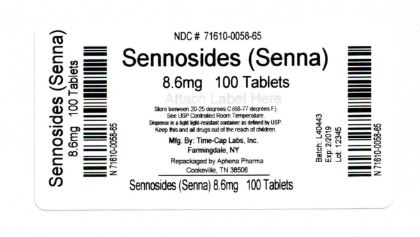
Count 8.6 mg 100 71610-058-65 Store between 20°-25°C (68°-77°F). See USP Controlled Room Temperature. Dispense in a tight light-resistant container as defined by USP. Keep this and all drugs out of the reach of children.
Repackaged by:

Cookeville, TN 38506
20180514JH - PRINCIPAL DISPLAY PANEL - 8.6 mg
-
INGREDIENTS AND APPEARANCE
SENEXON
senna tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 71610-058(NDC: 0536-5904) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES A and B (UNII: 1B5FPI42EN) (SENNOSIDES A and B - UNII:1B5FPI42EN) SENNOSIDES A and B 8.6 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) magnesium stearate (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) mineral oil (UNII: T5L8T28FGP) Product Characteristics Color brown Score no score Shape ROUND Size 9mm Flavor Imprint Code TCL080 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71610-058-65 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/04/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 02/09/2006 Labeler - Aphena Pharma Solutions - Tennessee, LLC (128385585) Establishment Name Address ID/FEI Business Operations Aphena Pharma Solutions - Tennessee, LLC 128385585 REPACK(71610-058)
Trademark Results [SENEXON]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SENEXON 76006874 2442626 Live/Registered |
THE HARVARD DRUG GROUP, L.L.C. 2000-03-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.