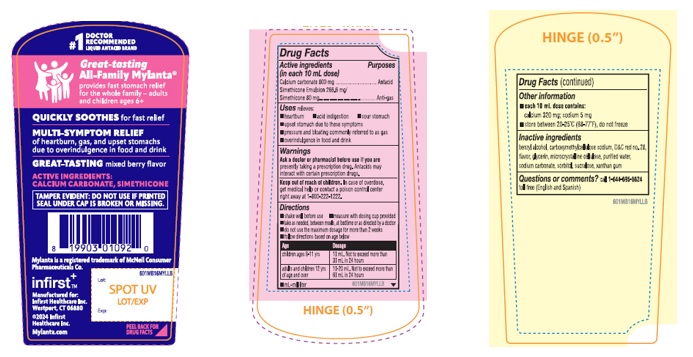
MYLANTA ALL-FAMILY MULTI SYMPTOM STOMACH REMEDY- calcium carbonate, simethicone liquid
Mylanta by
Drug Labeling and Warnings
Mylanta by is a Otc medication manufactured, distributed, or labeled by Infirst Healthcare Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
-
DOSAGE & ADMINISTRATION
Directions
shake well before use measure with dosing cup provided
take as needed, between meals, at bedtime or as directed by a doctor
do not use the maximum dosage for more than 2 weeks
follow directions based on age below
Age Dosage children ages 6-11 yrs 10 mL. Not to exceed more than 30 mL in 24 hours adults and children 12 yrs of age and over 10-20 mL. Not to exceed more than 60 mL in 24 hours mL=milliliter
- Other information
- INACTIVE INGREDIENT
- QUESTIONS
-
SPL UNCLASSIFIED SECTION
Great-tasting
All-Family Mylanta ®
QUICKLY SOOTHES for fast relief
MULTI-SYMPTOM RELIEF
of heartburn, gas, and upset stomachs
due to overindulgence in food and drink
GREAT-TASTING mixed berry flavor
ACTIVE INGREDIENTS:
CALCIUM CARBONATE, SIMETHICONE
TAMPER EVIDENT: DO NOT USE IF PRINTED SEAL UNDER CAP IS BROKEN OR MISSING.
infirst +™
Manufactured for:
Infirst Healthcare Inc.
Westport, CT 06880
2024 Infirst Healthcare Inc.
©Mylanta.com
Lot:
Exp:
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MYLANTA ALL-FAMILY MULTI SYMPTOM STOMACH REMEDY
calcium carbonate, simethicone liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 62372-520 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 800 mg in 10 mL DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 80 mg in 10 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) XANTHAN GUM (UNII: TTV12P4NEE) SODIUM CARBONATE (UNII: 45P3261C7T) CARBOXYMETHYLCELLULOSE CALCIUM (UNII: UTY7PDF93L) D&C RED NO. 28 (UNII: 767IP0Y5NH) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) GLYCERIN (UNII: PDC6A3C0OX) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color pink Score Shape Size Flavor BERRY (Mixed) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62372-520-16 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/21/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 05/21/2024 Labeler - Infirst Healthcare Inc. (079159739)
Trademark Results [Mylanta]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MYLANTA 86625249 not registered Dead/Abandoned |
McNeil Consumer Pharmaceuticals Co. 2015-05-11 |
 MYLANTA 74387798 1851270 Dead/Cancelled |
JOHNSON & JOHNSON-MERCK CONSUMER PHARMACEUTICALS COMPANY 1993-05-10 |
 MYLANTA 72115011 0720270 Live/Registered |
Stuart Company, The 1961-03-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.