NUTRILIPID I.V. FAT EMULSION- soybean oil injection, solution
NutriLipid I.V. Fat Emulsion by
Drug Labeling and Warnings
NutriLipid I.V. Fat Emulsion by is a Prescription medication manufactured, distributed, or labeled by B. Braun Medical Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NUTRILIPID® 20% safely and effectively. See full prescribing information for NUTRILIPID® 20%. NUTRILIPID® (lipid injectable emulsion), for intravenous useInitial U.S. Approval: 1975
WARNING: DEATH IN PRETERM INFANTS
See full prescribing information for complete
boxed warning- Deaths in preterm infants have been reported in literature. (5.1, 8.4)
- Autopsy findings included intravascular fat accumulation in the lungs. (5.1, 8.4)
- Preterm and low birth weight infants have poor clearance of intravenous lipid emulsion and increased free fatty acid plasma levels following lipid emulsion infusion. (5.1, 8.4)
INDICATIONS AND USAGE
Nutrilipid® 20% is indicated as a source of calories and essential fatty acids for parenteral nutrition and as a source of essential fatty acids when a deficiency occurs when oral or enteral nutrition is not possible, insufficient, or contraindicated (1)
DOSAGE AND ADMINISTRATION
- Nutrilipid® 20% Pharmacy Bulk Package is not intended for direct intravenous administration (2.1)
- For intravenous infusion only through a peripheral or central line (2.1)
- Recommended dosage depends on age, energy expenditure, clinical status, body weight, tolerance, ability to metabolize and consideration of additional energy given to the patient (2.4)
Age
Nutritional Requirements Initial Recommended Dosage Maximum Dosage Preterm and term infants (<1 year) 1 to 2 g/kg/day 3 g/kg/day Pediatric patients 1 to 10 years Pediatric patients 11 to <17 years 1 g/kg/day 2.5 g/kg/day Adults 1 to 1.5 g/kg/day 2.5 g/kg/day
DOSAGE FORMS AND STRENGTHS
Nutrilipid® 20% is a lipid injectable emulsion:
- Lipid content of 0.2 grams/mL in 250 mL, 350 mL, and 500 mL
- Lipid content of 0.2 grams/mL in 1000 mL Pharmacy Bulk Package (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
Adverse reactions have included hyperlipidemia, hypercoagulability, thrombophlebitis, thrombocytopenia (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact B. Braun Medical Inc. at 1-800-227-2862 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Coumarin and coumarin derivatives, including warfarin: Anticoagulant activity may be counteracted; monitor laboratory parameters (7.1)
USE IN SPECIFIC POPULATIONS
Hepatic Impairment: Use with caution; monitor liver function parameters (8.6)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2014
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: DEATH IN PRETERM INFANTS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Administration Instructions
2.2 Preparation Instructions for Nutrilipid® 20% Bags for Direct Infusion
2.3 Preparation Instructions for Admixing Using Total Parenteral Nutrition Pooling Bags
2.4 Dosing Considerations
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Death in Preterm Infants
5.2 Hypersensitivity Reactions
5.3 Infections
5.4 Fat Overload Syndrome
5.5 Refeeding Syndrome
5.6 Monitoring / Laboratory Tests
5.7 Interference with Laboratory Tests
5.8 Aluminum Toxicity
5.9 Risk of Parenteral Nutrition Associated Liver Disease
5.10 Hypertriglyceridemia
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Coumarin and Coumarin Derivatives
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: DEATH IN PRETERM INFANTS
- Deaths in preterm infants after infusion of intravenous lipid emulsions have been reported in the medical literature.
- Autopsy findings included intravascular fat accumulation in the lungs.
- Preterm infants and low birth weight infants have poor clearance of intravenous lipid emulsion and increased free fatty acid plasma levels following lipid emulsion infusion.
[see Warnings and Precautions (5.1) and Use in Specific Populations (8.4)]
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Administration Instructions
- Nutrilipid® 20% Pharmacy Bulk Package is not intended for direct intravenous administration.
- Nutrilipid® 20% is for intravenous infusion only through a peripheral or central line. When administered with dextrose and amino acids, the choice of a central or peripheral venous route should depend on the osmolarity of the final infusate.
- Use a non-vented infusion set or close the air vent on a vented set. Use of a vented intravenous administration set with the vent in the open position could result in air embolism.
- Use a dedicated line without any connections. Multiple connections could result in air embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is completed.
- Use a 1.2 micron in-line filter.
- Nutrilipid® 20% can be infused concurrently into the same vein as carbohydrate-amino acid solutions by means of a Y-connector located near the infusion site; flow rates of each solution should be controlled separately by infusion pumps.
- Do not use administration sets and lines that contain di-2-ethylhexyl phthalate (DEHP). Conventional administration sets contain polyvinyl chloride (PVC) components that have DEHP as a plasticizer.
2.2 Preparation Instructions for Nutrilipid® 20% Bags for Direct Infusion
Caution: Nutrilipid® 20% Pharmacy Bulk Package is not intended for direct intravenous administration.
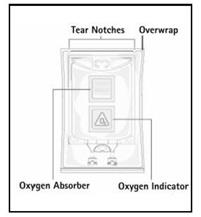
Step 1: Inspect infusion bag overwrap and primary bag and do not use if damaged. Inspect oxygen indicator and do not use if oxygen indicator is pink or dark pink. Use only if container and seals are intact.
Step 2: To open, tear overwrap starting from the tear notches (Figure 1). Remove Nutrilipid® 20% bag from overwrap and discard oxygen indicator, oxygen absorber and overwrap.
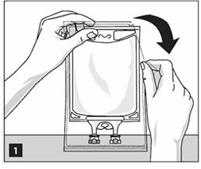
Step 3: Inspect Nutrilipid® 20% bag visually (Figure 2). Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Inspect Nutrilipid® 20% to ensure that the emulsion has not separated. Discard the bag if any particulates or discoloration are observed.
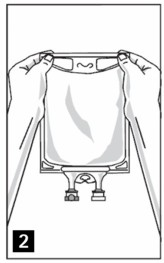
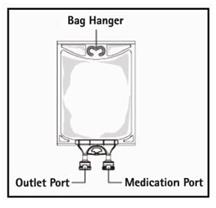
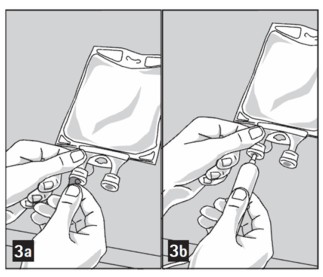
Step 4: Remove aluminum foil of outlet port at the bottom of the bag (Figure 3a) and attach administration set (Figure 3b).
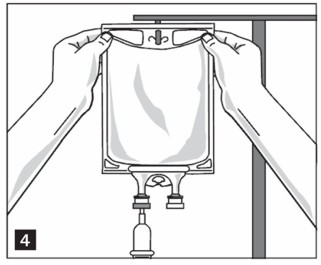
Step 5: Hang bag on IV Pole (Figure 4). If infusion pumps are used, flow rates of each parenteral fluid should be controlled with a separate pump.
Do not connect flexible bags in series to avoid air embolism due to possible residual gas contained in the primary bag.
Air embolism can result if residual gas in the bag is not fully evacuated prior to administration if the flexible bag is pressurized to increase flow rates.
If administration is controlled by a pumping device, discontinue pumping action before the bag runs dry to avoid air embolism.
Use of Admixtures in Nutrilipid® Infusion Bags for Direct Infusion
- Some additives may be incompatible and should not be used. If it is deemed advisable to introduce additives, prepare the admixture using strict aseptic techniques to avoid microbial contamination. Compatible supplemental medication (e.g., fat-soluble vitamins) may be added with a 19 to 22 gauge needle through the medication port. Additions to the bag should be evaluated by a pharmacist for compatibility. Questions about compatibility may be directed to B. Braun Medical Inc., Medical Affairs.
- The prime destabilizers of emulsions are excessive acidity (such as pH below 5) and inappropriate electrolyte content. Give careful consideration to additions of divalent cations (Ca++ and Mg++), which have been shown to cause emulsion instability.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Inspect Nutrilipid® 20% to ensure that:
- precipitates have not formed during the addition of additives and
- the emulsion has not separated during the addition of additives.
Discard the admixture if precipitates or discoloration are observed.
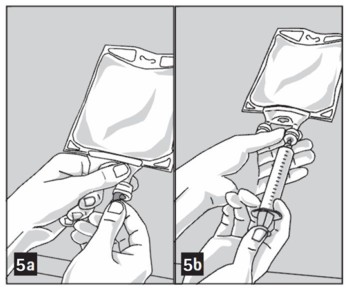
Step 1: Prepare the medication port by removal of aluminum foil (Figure 5a).
Step 2: Puncture the resealable medication port by using a 19 to 22 gauge needle and inject additive(s) (Figure 5b).
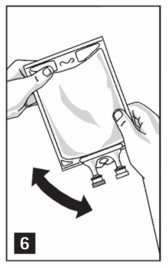
Step 3: Mix thoroughly when additives have been introduced (Figure 6).
Step 4: The medication port may be swabbed with disinfection agent (e.g., iso-propanol or chlorhexidine) before re-puncturing.
Step 5: Inspect emulsion bag visually for particulates or separation of the emulsion. Discard the bag if any particulates or discoloration are observed.
Do not store solutions containing additives.
2.3 Preparation Instructions for Admixing Using Total Parenteral Nutrition Pooling Bags
- Prepare the admixture into pooling bags using strict aseptic techniques to avoid microbial contamination.
- Do not add additives directly to Nutrilipid® 20% Pharmacy Bulk Package.
- Some additives may be incompatible and should not be used. If it is deemed advisable to introduce additives, prepare the admixture using strict aseptic techniques to avoid microbial contamination. Additions to the pooling bag should be evaluated by a pharmacist for compatibility. Questions about compatibility may be directed to B. Braun Medical Inc., Medical Affairs.
- Do not add Nutrilipid® 20% to the pooling bag first; destabilization of the lipid may occur from such an admixture.
- The following proper mixing sequence must be followed to minimize pH related problems by ensuring that typically acidic Dextrose Injections are not mixed with lipid emulsions alone:
- Transfer Dextrose Injection to the Total Parental Nutrition Pooling Bag
- Transfer Amino Acid Injection
- Transfer Nutrilipid® 20%
Use gentle agitation during admixing to minimize localized concentration effects; shake bags gently after each addition.
- Alternatively, simultaneous transfer to the pooling bag of Amino Acid Injection, Dextrose Injection and Nutrilipid® 20% is also permitted.
- The prime destabilizers of emulsions are excessive acidity (such as pH below 5) and inappropriate electrolyte content. Give careful consideration to additions of divalent cations (Ca++ and Mg++), which have been shown to cause emulsion instability. Amino acid solutions exert buffering effects that protect the emulsion.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Inspect Nutrilipid® 20% to ensure that:
- precipitates have not formed during the mixing or addition of additives and
- the emulsion has not separated. Separation of the emulsion can be visibly identified by a yellowish streaking or the accumulation of yellowish droplets in the admixed emulsion.
Discard the admixture if any of the above are observed.
- Use of the Pharmacy Bulk Package for admixing should be limited to up to four hours after opening.
- Admixtures should be used promptly with storage under refrigeration (2-8°C) not to exceed 24 hours and must be completely used within 24 hours after removal from refrigeration.
- Do not connect flexible bags in series to avoid air embolism due to possible residual gas contained in the primary bag.
- Air embolism can result if residual gas in the bag is not fully evacuated prior to administration if the flexible bag is pressurized to increase flow rates.
- If administration is controlled by a pumping device, discontinue pumping action before the bag runs dry to avoid air embolism.
2.4 Dosing Considerations
The dosing of Nutrilipid® 20% depends on the patient’s individual energy requirements, influenced by body weight, tolerance, clinical status, age-related growth rate in pediatric patients, and the ability to eliminate and metabolize fat.
For partial parenteral nutrition, energy supplied by oral or enteral nutrition has to be taken into account. For complete parenteral nutrition, concomitant supplementation with amino acids, carbohydrates, electrolytes, vitamins, and trace elements is necessary.
Prior to administration of Nutrilipid® 20%, correct severe water and electrolyte disorders, severe fluid overload states, and severe metabolic disorders. Before starting the infusion, obtain serum triglyceride levels to establish the baseline value.
Recommended Adult and Pediatric Dosing
The recommended nutritional requirements of fat and recommended dosages of Nutrilipid® 20% to be administered to meet those requirements for adults and pediatric patients are provided in Table 1, along with recommendations for the initial and maximum infusion rates. The recommended duration of infusion for Nutrilipid® 20% is between 12 and 24 hours, depending on the clinical situation. Adjust the administration flow rate by taking into account the dose being administered, the daily volume/intake, and the duration of the infusion [see Overdosage (10)].
Treatment with parenteral nutrition may be continued for as long as is required by the patient’s condition.
In patients with elevated triglyceride levels, initiate Nutrilipid® 20% at a lower dosage, and advance in smaller increments, monitoring the triglyceride levels with each adjustment [see Warnings and Precautions (5.6) and (5.10)].
When Nutrilipid® 20% is administered to correct essential fatty acid deficiency, 8% to 10% of the caloric input should be supplied by Nutrilipid® 20% in order to provide adequate amounts of linoleic and linolenic acids.
Table 1: Adult and Pediatric Nutritional Requirements1,2 as Provided by Nutrilipid® 20% and Infusion Rate for Direct Infusion Only
- *
Nutrilipid® 20% Pharmacy Bulk Package is not intended for direct intravenous administration.
- †
Daily dose should also not exceed a maximum of 60% of total energy requirements [see Overdosage (10)].
Age Nutritional Requirements Direct Infusion Rate* Recommended Initial Dosage and Maximum Dosage Initial Maximum Preterm and term infants (less than 1 year of age)
Initial 1 to 2 g/kg/day
not to exceed 3 g/kg/day†
0.05 mL/min for the first 10 to 15 minutes; gradually increase to the required rate after 15 minutes 0.75 mL/kg/hour Pediatric patients 1 to 10 years of age Initial 1 to 2 g/kg/day
not to exceed 3 g/kg/day†
0.75 mL/kg/hour Pediatric patients 11 to <17 years of age Initial 1 g/kg/day
not to exceed 2.5 g/kg/day†
0.5 mL/kg/hour Adults 1 to 1.5 g/kg/day
not to exceed 2.5 g/kg/day†
0.5 mL/min for the first 15 to 30 minutes; gradually increase to the required rate after 30 minutes 0.5 mL/kg/hour - 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Nutrilipid® 20% is contraindicated in patients who have:
- Known hypersensitivity to egg or soybean proteins or to any of the ingredients, including excipients, or
- Severe hyperlipidemia (serum triglyceride concentrations above 1000 mg/dL) or severe disorders of lipid metabolism characterized by hypertriglyceridemia.
-
5 WARNINGS AND PRECAUTIONS
5.1 Death in Preterm Infants
Deaths in preterm infants after infusion of intravenous lipid emulsions have been reported. Autopsy findings included intravascular lipid accumulation in the lungs.
Base the decision to treat preterm and small for gestational age infants with intravenous lipid emulsion upon careful benefit-risk assessment. Strictly adhere to the recommended total daily dose; hourly infusion rate should be as slow as possible and should not exceed 0.75 mL/kg/hour [see Dosage and Administration (2.4)].
Preterm and small for gestational age infants have poor clearance of intravenous lipid emulsion and increased free fatty acid plasma levels following fat emulsion infusion; therefore, seriously consider administration of less than the maximum recommended doses in these patients in order to decrease the likelihood of intravenous fat overload.
Carefully monitor the infant’s ability to eliminate the infused lipids from the circulation (such as serum triglycerides and/or plasma free fatty acid levels) [see Warnings and Precautions (5.10)].
Because of the risk of thrombocytopenia, monitor platelet counts frequently in neonatal patients receiving parenteral nutrition with Nutrilipid® 20%.
5.2 Hypersensitivity Reactions
Stop infusion immediately and treat patient accordingly if signs or symptoms of a hypersensitivity or allergic reaction develop. Signs or symptoms may include: tachypnea, dyspnea, hypoxia, bronchospasm, tachycardia, hypotension, cyanosis, vomiting, nausea, headache, sweating, dizziness, altered mentation, flushing, rash, urticaria, erythema, pyrexia, and chills.
5.3 Infections
Patients who require parenteral nutrition are at high risk of infections due to malnutrition and their underlying disease state.
Infection and sepsis may occur as a result of the use of intravenous catheters to administer parenteral nutrition, poor maintenance of catheters, or immunosuppressive effects of illness, drugs, and parenteral formulations.
Decrease the risk of septic complications with heightened emphasis on aseptic technique in catheter placement and maintenance, as well as aseptic technique in the preparation of the nutritional formula.
Carefully monitor for signs and symptoms (including fever and chills) of early infections, including laboratory test results (including leukocytosis and hyperglycemia) and frequent checks of the parenteral access device.
5.4 Fat Overload Syndrome
Fat overload syndrome is a rare condition that has been reported with intravenous lipid formulations. A reduced or limited ability to metabolize the lipids contained in Nutrilipid® 20% accompanied by prolonged plasma clearance may result in a syndrome characterized by a sudden deterioration in the patient's condition accompanied by fever, anemia, leukopenia, thrombocytopenia, coagulation disorders, hyperlipidemia, liver fatty infiltration (hepatomegaly), deteriorating liver function, and central nervous system manifestations (e.g., coma). The cause of the fat overload syndrome is unclear. The syndrome is usually reversible when the infusion of the lipid emulsion is stopped. Although it has been most frequently observed when the recommended lipid dose was exceeded, cases have also been described where the lipid formulation was administered according to instructions.
5.5 Refeeding Syndrome
Refeeding severely undernourished patients with parenteral nutrition may result in the refeeding syndrome, characterized by the intracellular shift of potassium, phosphorus, and magnesium as the patient becomes anabolic. Thiamine deficiency and fluid retention may also develop. Carefully monitor severely undernourished patients and slowly increase their nutrient intakes, while avoiding overfeeding, to prevent these complications.
5.6 Monitoring / Laboratory Tests
Monitor fluid status closely in patients with pulmonary edema or heart failure.
Monitor serum triglycerides [see Warnings and Precautions (5.10)], fluid and electrolyte status, serum osmolarity, blood glucose, liver and kidney function, blood count (including platelets), and coagulation parameters throughout treatment.
5.7 Interference with Laboratory Tests
Content of Vitamin K may counteract anticoagulant activity [see Drug Interactions (7)].
The lipids contained in this emulsion may interfere with the results of certain laboratory tests if the blood sample is taken before the lipids are eliminated from the serum (these are generally eliminated after a period of 5 to 6 hours without receiving lipids).
5.8 Aluminum Toxicity
Nutrilipid® 20% contains no more than 25 mcg/L of aluminum.
The aluminum contained in Nutrilipid® 20% may reach toxic levels with prolonged administration in patients with impaired kidney function. Preterm infants are at greater risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions that contain aluminum.
Patients with impaired kidney function, including preterm infants, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day, accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration of total parenteral nutrition products.
5.9 Risk of Parenteral Nutrition Associated Liver Disease
Parenteral Nutrition Associated Liver Disease (PNALD) has been reported in patients who receive parenteral nutrition for extended periods of time, especially preterm infants, and can present as cholestasis or steatohepatitis . The exact etiology is unknown and is likely multifactorial. Intravenously administered phytosterols (plant sterols) contained in plant-derived lipid formulations have been associated with development of PNALD although a causal relationship has not been clearly established.
If Nutrilipid® 20% treated patients develop liver test abnormalities consider discontinuation or dose reduction.
5.10 Hypertriglyceridemia
To evaluate the patient’s capacity to eliminate and metabolize the infused lipid emulsion, measure serum triglycerides before the start of infusion (baseline value), with each increase in dosage, and regularly throughout treatment.
Reduce dose of Nutrilipid® 20% and monitor serum triglyceride levels in patients with serum triglyceride concentrations above 400 mg/dL to avoid the clinical consequences associated with hypertriglyceridemia. Serum triglyceride levels above 1000 mg/dL have been associated with an increased risk of pancreatitis.
Impaired lipid metabolism with hypertriglyceridemia may occur in conditions such as inherited lipid disorders, obesity, diabetes mellitus, and metabolic syndrome. In these cases, increased triglycerides can also be increased by glucose and/or overfeeding. Monitor overall energy intake and other sources of fat and glucose, as well as drugs that may interfere with lipid and glucose metabolism.
-
6 ADVERSE REACTIONS
Adverse Reactions described elsewhere in labeling:
- Death in Preterm Infants [see Warnings and Precautions (5.1)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.2)]
- Infections [see Warnings and Precautions (5.3)]
- Fat Overload Syndrome [see Warnings and Precautions (5.4)]
- Refeeding Syndrome [see Warnings and Precautions (5.5)]
- Aluminum Toxicity [see Warnings and Precautions (5.8)]
- Risk of Parenteral Nutrition Associated Liver Disease [see Warnings and Precautions (5.9)]
- Hypertriglyceridemia [see Warnings and Precautions (5.10)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse reactions reported with other intravenous lipid emulsions include hyperlipidemia, hypercoagulability, thrombophlebitis, and thrombocytopenia.
Adverse reactions reported in long-term use with other intravenous lipid emulsions include hepatomegaly, jaundice due to central lobular cholestasis, splenomegaly, thrombocytopenia, leukopenia, abnormalities in liver function tests, brown pigmentation of the liver and overloading syndrome (focal seizures, fever, leukocytosis, hepatomegaly, splenomegaly and shock).
-
7 DRUG INTERACTIONS
7.1 Coumarin and Coumarin Derivatives
The soybean oil present in Nutrilipid® 20% has vitamin K1. Vitamin K can reverse the anticoagulant activity of coumarin and coumarin derivatives, including warfarin, which work by blocking recycling of vitamin K. Monitor laboratory parameters for anticoagulant activity in patients who are on both Nutrilipid® 20% and coumarin or coumarin derivatives.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C
Risk Summary
There are no adequate or well controlled studies with Nutrilipid® 20% in pregnant women. Additionally, animal reproduction studies have not been conducted with Nutrilipid® 20%. It is not known whether Nutrilipid® 20% can cause fetal harm when administered to a pregnant woman. Nutrilipid® 20% should be given to a pregnant woman only if clearly needed.
8.3 Nursing Mothers
It is not known whether Nutrilipid® 20% is present in human milk. Because many drugs are present in human milk, caution should be exercised when Nutrilipid® 20% is administered to a nursing woman.
8.4 Pediatric Use
The evidence for safety and efficacy in pediatric patients of Nutrilipid® 20% as a source of calories and essential fatty acids for parenteral nutrition and as a source of essential fatty acids when a deficiency occurs when oral or enteral nutrition is not possible, insufficient, or contraindicated is derived from the published literature and clinical experience with similar soybean oil based intravenous lipid emulsions.
Deaths in preterm infants after infusion of intravenous lipid emulsion have been reported [see Warnings and Precautions (5.1)]. Patients, particularly preterm infants, are at risk for aluminum toxicity [see Warnings and Precautions (5.8)]. Patients, including pediatric patients, may be at risk for PNALD [see Warnings and Precautions (5.9)]. In clinical trials of a pure soybean oil based intravenous lipid emulsion product, thrombocytopenia in neonates occurred (less than 1%).
8.5 Geriatric Use
Clinical studies of Nutrilipid® 20% did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
Parenteral nutrition should be used with caution in patients with hepatic impairment. Hepatobiliary disorders are known to develop in some patients without preexisting liver disease who receive parenteral nutrition, including cholestasis, hepatic steatosis, fibrosis and cirrhosis (parenteral nutrition associated liver disease), possibly leading to hepatic failure. Cholecystitis and cholelithiasis have also been observed. The etiology of these disorders is thought to be multifactorial and may differ between patients.
Monitor liver function parameters closely. Patients developing signs of hepatobiliary disorders should be assessed early by a clinician knowledgeable in liver diseases in order to identify causative and contributory factors, and possible therapeutic and prophylactic interventions.
-
10 OVERDOSAGE
In the event of overdose, fat overload syndrome may result [see Warnings and Precautions (5.4)]. Stop the infusion to allow lipids to clear from serum. The effects are usually reversible after the lipid infusion is stopped. If medically appropriate, further intervention may be indicated. The lipid administered and fatty acids produced are not dialyzable.
-
11 DESCRIPTION
Nutrilipid® 20% is a sterile, nonpyrogenic fat emulsion prepared for intravenous administration.
Each 100 mL of Nutrilipid® 20% contains: Soybean Oil 20 g; Egg Yolk Phospholipid 1.2 g; Glycerin USP (glycerol) 2.5 g; Sodium Oleate 0.03 g; Water for Injection USP qs.
pH adjusted with Sodium Hydroxide NF.
pH: 6.8 (6.0-8.9); Osmolality: 390 mOsmolKg (actual). Contains emulsified fat particles averaging approximately 0.26 micron in diameter, similar to naturally occurring chylomicrons. The total caloric value, including fat, phospholipid, and glycerol is 2.0 Kcal per mL.
Soybean oil is a refined natural product consisting of a mixture of neutral triglycerides of predominantly unsaturated fatty acids with the following structure:
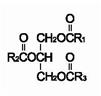
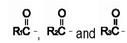 are saturated and unsaturated fatty acid residues. The major component fatty acids are linoleic (48% - 58%), oleic (17% - 30%), palmitic (9% -13%), linolenic (4% - 11%), and stearic (2.5% - 5.0%). These fatty acids have the folIowing chemical and structural formulas:
are saturated and unsaturated fatty acid residues. The major component fatty acids are linoleic (48% - 58%), oleic (17% - 30%), palmitic (9% -13%), linolenic (4% - 11%), and stearic (2.5% - 5.0%). These fatty acids have the folIowing chemical and structural formulas:Linoleic Acid
C18H32O2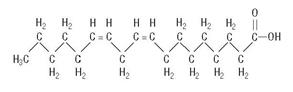
Oleic Acid
C18H34O2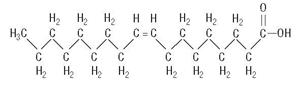
Palmitic Acid
C16H32O2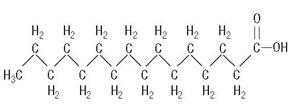
Linolenic Acid
C18H30O2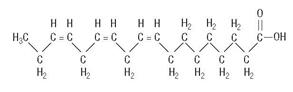
Stearic Acid
C18H36O2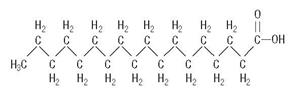
Egg yolk phospholipids are a mixture of naturally occurring phospholipids isolated from egg yolk.
Glycerol is chemically designated C3H803 and is a clear colorless, hygroscopic syrupy liquid. It is added to adjust tonicity.
Not made with natural rubber latex, PVC or DEHP.
Drug product contains no more than 25 mcg/L of aluminum.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Nutrilipid® 20% administered intravenously provides biologically utilizable source of calories and essential fatty acids.
Fatty acids serve as an important substrate for energy production. The most common mechanism of action for energy production derived from fatty acid metabolism is beta oxidation. Fatty acids are important for membrane structure and function, precursors for bioactive molecules (such as prostaglandins), and as regulators of gene expression.
Nutrilipid® 20% causes an increase in heat production, decrease in respiratory quotient, and increase in oxygen consumption following its administration.
12.3 Pharmacokinetics
The infused lipid particles are removed from the bloodstream in a manner generally thought to be similar to the enzymatic clearance of naturally produced chylomicrons formed after enteral fat intake. Following infusion, there is a transient increase in plasma triglycerides. The triglycerides are hydrolyzed to free fatty acids and glycerol by the enzyme lipoprotein lipase. The free fatty acids either enter the tissues (where they may be oxidized or resynthesized into triglycerides and stored) or circulate in the plasma, bound to albumin. In the liver, circulating free fatty acids are oxidized or converted to very low density lipoproteins that re-enter the bloodstream.
Phosphatides are the hydrophobic components of membranes and provide electrically insulated layers. They are involved in the formation of membrane structures. Choline prevents deposition of fat in the liver.
Glycerol is metabolized to carbon dioxide and glycogen or is used in the synthesis of body fats.
- 13 NONCLINICAL TOXICOLOGY
-
15 REFERENCES
- Carney LN, Nepa A, Cohen SS, Dean A, Yanni C, Markowitz G. Chapter 34. Parenteral and enteral nutrition support: Determining the best way to feed. IN: The A.S.P.E.N. Pediatric Nutrition Support Core Curriculum. Corkins MR (editor in chief). American Society for Parenteral and Enteral Nutrition, Silver Spring, MD: 2010, pages 433-447.
- Mirtallo J, Canada T, Johnson D, Kumpf V, Petersen C, Sacks G, et al. Task Force for the Revision of Safe Practices for Parenteral Nutrition, Special Report: safe practices for parenteral nutrition. JPEN J Parenter Enteral Nutr: 2004, 28 (6 Suppl).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Nutrilipid® 20% is supplied as a sterile emulsion in the following fill sizes:
NDC REF Volume 0264-4460-00 S4600 1000 mL in Pharmacy Bulk Package container 0264-4460-10 S4601 500 mL 0264-4460-20 S4602 350 mL in 500 mL container 0264-4460-30 S4603 250 mL Do not freeze. If accidentally frozen, discard the container. Store below 25°C (77°F).
Use of the Pharmacy Bulk Package for admixing should be limited to up to four hours after opening. Admixtures should be used promptly with storage under refrigeration (2-8°C) not to exceed 24 hours and must be completely used within 24 hours after removal from refrigeration.
-
17 PATIENT COUNSELING INFORMATION
Inform patients of the following:
- Deaths in preterm infants after infusion of intravenous lipid emulsions such as Nutrilipid® 20% have been reported. Blood tests will be performed during treatment to monitor for the infant’s ability to eliminate the infused lipids.
- Laboratory monitoring throughout treatment may be necessary. Advise patients to comply with periodic laboratory tests and routine follow up with their healthcare provider.
- Allergic reactions to Nutrilipid® 20% can occur. Advise patients to contact their healthcare provider if they experience any signs or symptoms of an allergic reaction, including wheezing, swelling of the lips, tongue or throat, an itchy rash, difficulty breathing, rapid heart rate or feeling faint.
- Risk of infection and sepsis is increased with Nutrilipid® 20% and other products administered through an intravenous catheter. Advise patients to contact their healthcare provider if they develop any signs or symptoms of infection including redness extended from the injection site, fever or chills.
If Nutrilipid® 20% is administered by the patient at home, also instruct patients:
- Not to deviate from the administration instructions given by the healthcare provider.
- Inspect the bag visually for particulate matter and to ensure the lipid emulsion is an evenly distributed liquid with a milky appearance with no visible oil droplets at the surface prior to administration. Discard the bag and contact the healthcare provider if particulates or discoloration are observed.
- Ensure that there is an in-line filter in place prior to and during administration.
- Use a non-vented infusion set or close the air vent on a vented set. Use of a vented intravenous administration set with the vent in the open position could result in air embolism.
- If administration is controlled by a pumping device, discontinue pumping action before the bag runs dry to avoid air embolism.
- Any remaining product from partially used bag must be discarded.
- Inform their healthcare provider about any changes in prescription or over-the-counter medications and supplements.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 1000 mL
Nutrilipid™ 20% I.V. Fat Emulsion
REF S4600
NDC: 0264-4460-00LOT
EXP:
SN:1000 mL
For Intravenous Use
Each 100 mL contains:
Soybean Oil 20 g
Egg Phospholipids, USP 1.2 g
Sodium Oleate 0.03 g
Glycerin, USP 2.5 g
Water for Injection q.s.Pharmacy Bulk Package
Not For Direct Infusion
Once container closure has been penetrated, the emulsion should be used without delay. See package insert for proper use. Contains no more than 25 mcg/L of aluminum.
Sterile.
Do not store above 25°C (77°F). Avoid excessive heat. Do not freeze Nutrilipid 20% I.V. Fat Emulsion. If accidentally frozen, discard the bag.
Do not use if there appears to be any separation of the emulsion.
Not made with natural rubber latex, DEHP or PVC.
Rx only

Manufactured for
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
Made in GermanySET
370/12615443/0814

-
PRINCIPAL DISPLAY PANEL - 500 mL
Nutrilipid™ 20% I.V. Fat Emulsion
REF S4601
NDC: 0264-4460-10LOT
EXP:
SN:500 mL
For Intravenous Use
Each 100 mL contains:
Soybean Oil 20 g
Egg Phospholipids, USP 1.2 g
Sodium Oleate 0.03 g
Glycerin, USP 2.5 g
Water for Injection q.s.Administer intravenously.
Once container closure has been penetrated, the emulsion should be used without delay. See package insert for proper use. Contains no more than 25 mcg/L of aluminum. Sterile. Do not store above 25°C (77°F). Avoid excessive heat. Do not freeze Nutrilipid 20% I.V. Fat Emulsion. If accidentally frozen, discard the bag. Do not use if there appears to be any separation of the emulsion.
Not made with natural rubber latex, DEHP, or PVC.
Rx only

Manufactured for
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
Made in GermanySET
ADD370/12615441/0714
-
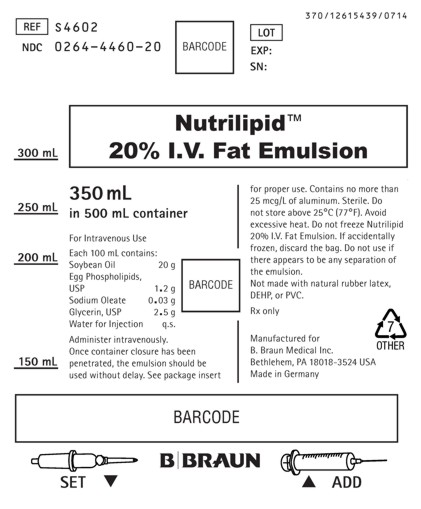
PRINCIPAL DISPLAY PANEL - 350 mL
Nutrilipid™ 20% I.V. Fat Emulsion
REF S4602
NDC: 0264-4460-20LOT
EXP:
SN:350 mL in 500 mL container
For Intravenous Use
Each 100 mL contains:
Soybean Oil 20 g
Egg Phospholipids, USP 1.2 g
Sodium Oleate 0.03 g
Glycerin, USP 2.5 g
Water for Injection q.s.Administer intravenously.
Once container closure has been penetrated, the emulsion should be used without delay. See package insert for proper use. Contains no more than 25 mcg/L of aluminum. Sterile. Do not store above 25°C (77°F). Avoid excessive heat. Do not freeze Nutrilipid 20% I.V. Fat Emulsion. If accidentally frozen, discard the bag. Do not use if there appears to be any separation of the emulsion.
Not made with natural rubber latex, DEHP, or PVC.
Rx only

Manufactured for
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
Made in GermanySET
ADD370/12615439/0714
-
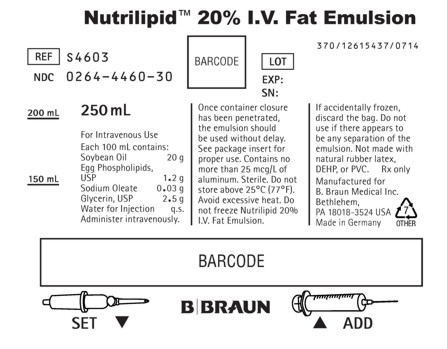
PRINCIPAL DISPLAY PANEL - 250 mL
Nutrilipid™ 20% I.V. Fat Emulsion
REF S4603
NDC: 0264-4460-30LOT
EXP:
SN:250 mL
For Intravenous Use
Each 100 mL contains:
Soybean Oil 20 g
Egg Phospholipids, USP 1.2 g
Sodium Oleate 0.03 g
Glycerin, USP 2.5 g
Water for Injection q.s.Administer intravenously.
Once container closure has been penetrated, the emulsion should be used without delay. See package insert for proper use. Contains no more than 25 mcg/L of aluminum. Sterile. Do not store above 25°C (77°F). Avoid excessive heat. Do not freeze Nutrilipid 20% I.V. Fat Emulsion. If accidentally frozen, discard the bag. Do not use if there appears to be any separation of the emulsion. Not made with natural rubber latex, DEHP, or PVC.
Rx only

Manufactured for
B. Braun Medical Inc.
Bethlehem, PA 18018-3524 USA
Made in GermanySET
ADD370/12615437/0714
-
INGREDIENTS AND APPEARANCE
NUTRILIPID I.V. FAT EMULSION
soybean oil injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0264-4460 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SOYBEAN OIL (UNII: 241ATL177A) (SOYBEAN OIL - UNII:241ATL177A) SOYBEAN OIL 20 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) 1.2 g in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 2.5 g in 100 mL SODIUM OLEATE (UNII: 399SL044HN) 0.03 g in 100 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0264-4460-10 12 in 1 CASE 06/30/2014 1 1 in 1 CARTON 1 500 mL in 1 CONTAINER; Type 0: Not a Combination Product 2 NDC: 0264-4460-20 12 in 1 CASE 06/30/2014 2 1 in 1 CARTON 2 500 mL in 1 CONTAINER; Type 0: Not a Combination Product 3 NDC: 0264-4460-30 12 in 1 CASE 06/30/2014 3 1 in 1 CARTON 3 250 mL in 1 CONTAINER; Type 0: Not a Combination Product 4 NDC: 0264-4460-00 8 in 1 CASE 06/30/2014 4 1 in 1 CARTON 4 1000 mL in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019531 06/30/2014 Labeler - B. Braun Medical Inc. (002397347)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.