BLISTEX EUROPEAN FORMULA- lanolin, octinoxate, and oxybenzone paste
Blistex by
Drug Labeling and Warnings
Blistex by is a Otc medication manufactured, distributed, or labeled by Blistex Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
-
Inactive ingredients
aloe barbadensis leaf extract, camphor, cetyl alcohol, cocoyl hydrolyzed soy protein, euphorbia cerifera (candelilla) wax, fragrance, isostearoyl hydrolyzed collagen, menthol, microcrystalline wax, olea europaea (olive) fruit oil, petrolatum, saccharin, theobroma cacao (cocoa) seed butter, thymol, tocopheryl acetate, vanillin, vitis vinifera (grape) seed oil
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 7 g Container Label
NDC: 10157-9539-1
Blistex®
LIP PROTECTANT/
SUNSCREENSPF
15EUROPEAN FORMULA
LIP CONDITIONER™Moisturizes & Protects For Healthy, Beautiful Lips
Enriched with Olive & Grapeseed Oils,
Vitamin E, Cocoa Butter, Lanolin and CollagenNET WT. .25 oz (7g)
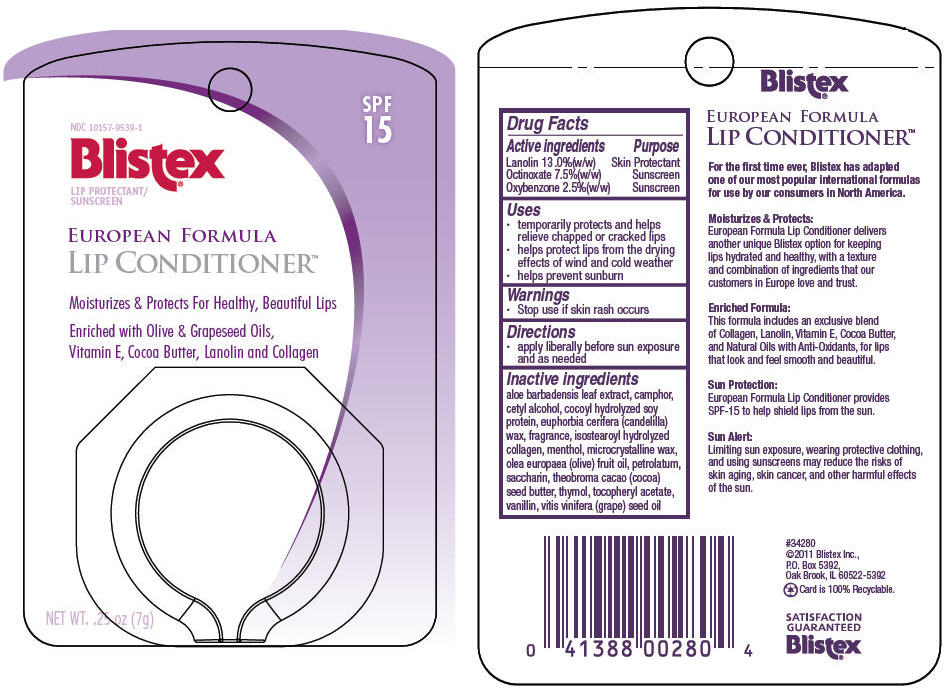
-
INGREDIENTS AND APPEARANCE
BLISTEX EUROPEAN FORMULA
lanolin, octinoxate, and oxybenzone pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 10157-9539 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Lanolin (UNII: 7EV65EAW6H) (Lanolin - UNII:7EV65EAW6H) Lanolin 13 g in 100 g Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 7.5 g in 100 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 2.5 g in 100 g Inactive Ingredients Ingredient Name Strength aloe vera leaf (UNII: ZY81Z83H0X) camphor (synthetic) (UNII: 5TJD82A1ET) cetyl alcohol (UNII: 936JST6JCN) candelilla wax (UNII: WL0328HX19) menthol (UNII: L7T10EIP3A) microcrystalline wax (UNII: XOF597Q3KY) olive oil (UNII: 6UYK2W1W1E) petrolatum (UNII: 4T6H12BN9U) saccharin (UNII: FST467XS7D) cocoa butter (UNII: 512OYT1CRR) thymol (UNII: 3J50XA376E) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) vanillin (UNII: CHI530446X) grape seed oil (UNII: 930MLC8XGG) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10157-9539-1 1 in 1 BLISTER PACK 1 7 g in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 07/01/2011 Labeler - Blistex Inc. (005126354) Establishment Name Address ID/FEI Business Operations Blistex Inc. 005126354 MANUFACTURE(10157-9539)
Trademark Results [Blistex]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BLISTEX 88143446 5924118 Live/Registered |
Blistex Inc. 2018-10-04 |
 BLISTEX 78233453 2817472 Live/Registered |
BLISTEX INC. 2003-04-03 |
 BLISTEX 74421493 1897266 Dead/Cancelled |
Blistex Bracken Limited Partnership 1993-08-04 |
 BLISTEX 73541344 1373396 Live/Registered |
BLISTEX BRACKEN LIMITED PARTNERSHIP 1985-06-05 |
 BLISTEX 73136745 1094204 Live/Registered |
BRACKEN, JIM L. 1977-08-08 |
 BLISTEX 72226892 0804558 Live/Registered |
BLISTEX COMPANY 1965-09-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.