MYMEDIC SUNSCREEN- homosalate, titanium dioxide, ethylhexyl salicylate, phenylbenzimidazole sulfonic acid patch
MyMedic Sunscreen by
Drug Labeling and Warnings
MyMedic Sunscreen by is a Otc medication manufactured, distributed, or labeled by Bath Concept Cosmetics (Dongguan) Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- DOSAGE & ADMINISTRATION
- WARNINGS
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
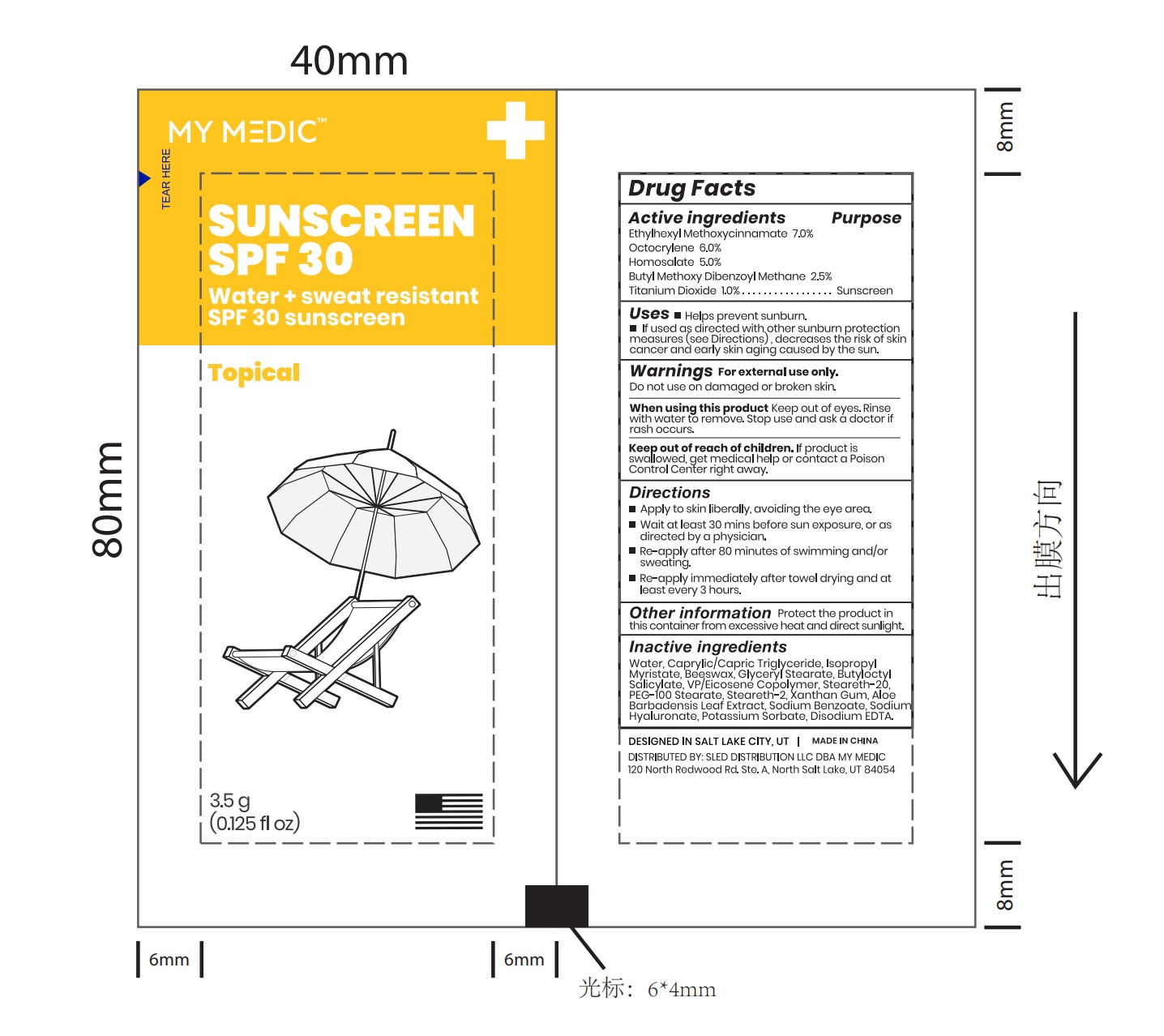
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MYMEDIC SUNSCREEN
homosalate, titanium dioxide, ethylhexyl salicylate, phenylbenzimidazole sulfonic acid patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 61805-118 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIBENZOYLMETHANE (UNII: ANS7ME8OKC) (DIBENZOYLMETHANE - UNII:ANS7ME8OKC) DIBENZOYLMETHANE 2.5 mg in 100 g ETHYL METHOXYCINNAMATE (UNII: SD418S06XD) (ETHYL METHOXYCINNAMATE - UNII:SD418S06XD) ETHYL METHOXYCINNAMATE 7 g in 100 g TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1 g in 100 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 5 g in 100 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 6 g in 100 g Inactive Ingredients Ingredient Name Strength BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) ALOE (UNII: V5VD430YW9) PEG-100 STEARATE (UNII: YD01N1999R) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) PEG-8 BEESWAX (UNII: 3C1QUF1TIR) XANTHAN GUM (UNII: TTV12P4NEE) STEARETH-2 (UNII: V56DFE46J5) WATER (UNII: 059QF0KO0R) STEARETH-20 (UNII: L0Q8IK9E08) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) Product Characteristics Color Score Shape Size 40mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61805-118-01 3.5 g in 1 POUCH; Type 0: Not a Combination Product 06/04/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/04/2024 Labeler - Bath Concept Cosmetics (Dongguan) Co., Ltd (529623933) Registrant - Bath Concept Cosmetics (Dongguan) Co., Ltd (529623933) Establishment Name Address ID/FEI Business Operations Bath Concept Cosmetics (Dongguan) Co., Ltd 529623933 manufacture(61805-118)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.