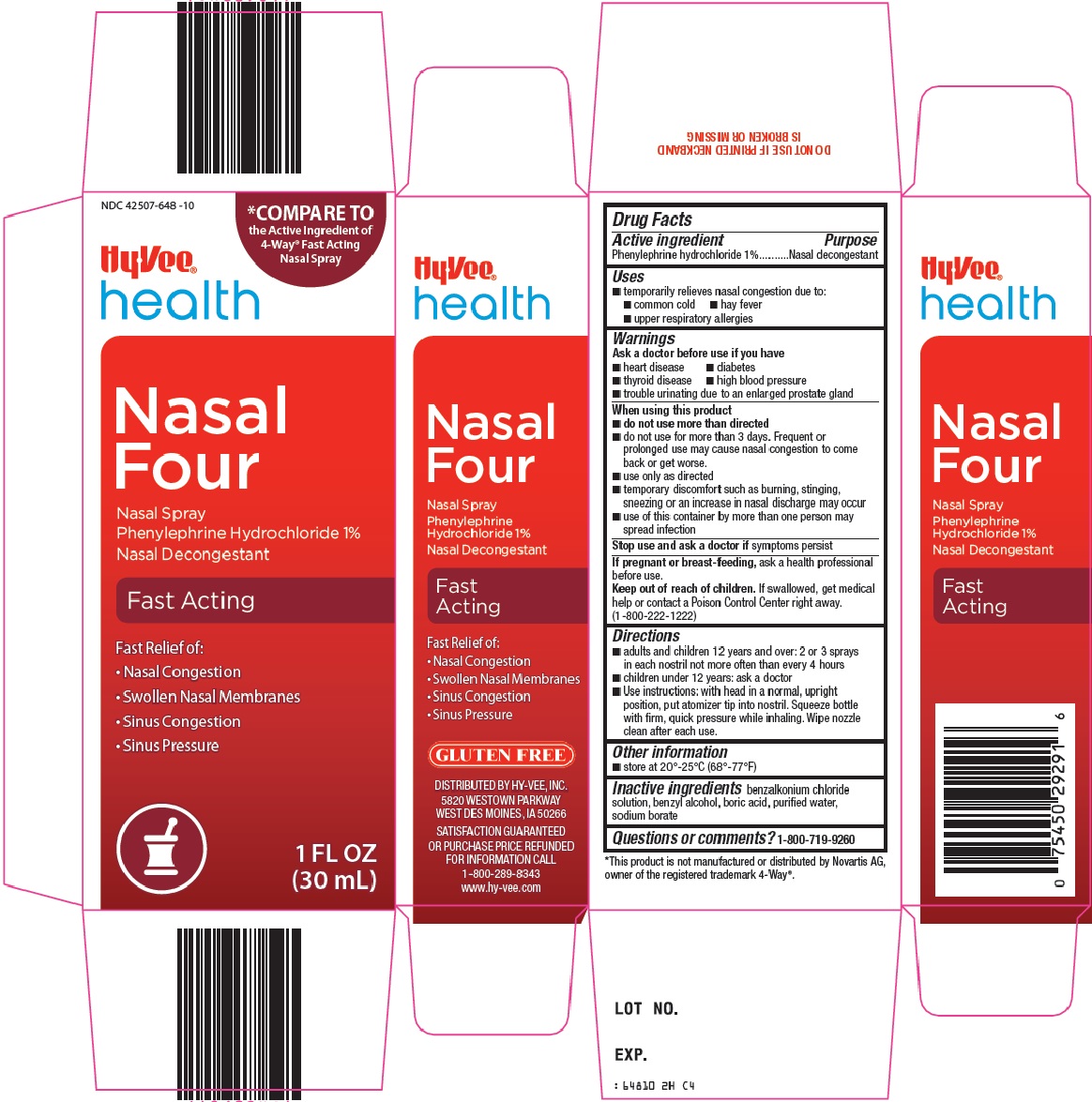
Hy-Vee, Inc. Nasal Four Drug Facts
Nasal Four by
Drug Labeling and Warnings
Nasal Four by is a Otc medication manufactured, distributed, or labeled by HyVee Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
NASAL FOUR- phenylephrine hydrochloride spray
HyVee Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Hy-Vee, Inc. Nasal Four Drug Facts
Uses
- temporarily relieves nasal congestion due to:
- common cold
- hay fever
- upper respiratory allergies
Ask a doctor before use if you have
- heart disease
- diabetes
- thyroid disease
- high blood pressure
- trouble urinating due to an enlarged prostate gland
When using this product
- do not use more than directed
- do not use for more than 3 days. Frequent or prolonged use may cause nasal congestion to come back or get worse.
- use only as directed
- temporary discomfort such as burning, stinging, sneezing or an increase in nasal discharge may occur
- use of this container by more than one person may spread infection
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Directions
- adults and children 12 years and over: 2 or 3 sprays in each nostril not more often than every 4 hours
- children under 12 years: ask a doctor
- Use instructions: with head in a normal, upright position, put atomizer tip into nostril. Squeeze bottle with firm, quick pressure while inhaling. Wipe nozzle clean after each use.
| NASAL FOUR
phenylephrine hydrochloride spray |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - HyVee Inc (006925671) |
Revised: 11/2019
Document Id: 7bf6272e-89c2-4ef2-a925-709ebcf022aa
Set id: 19879fc8-4962-4c6e-87ed-b874d1d564af
Version: 3
Effective Time: 20191122
HyVee Inc