antibacterial hand sanitizer
antibacterial hand sanitizer by
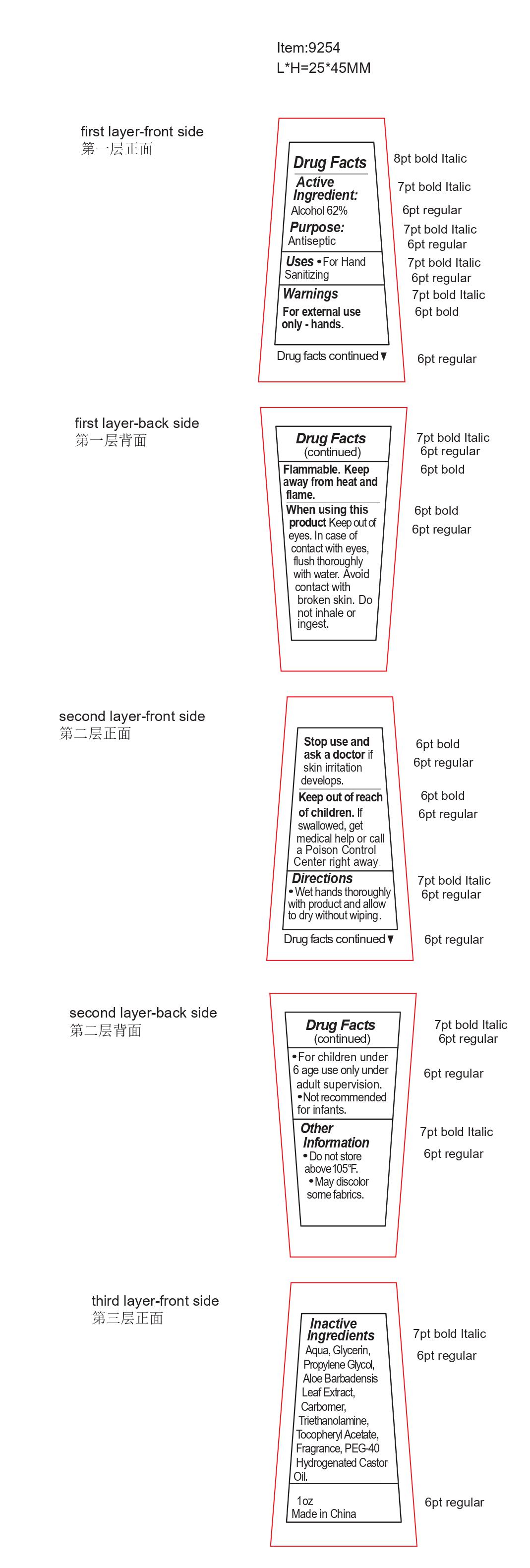
Drug Labeling and Warnings
antibacterial hand sanitizer by is a Otc medication manufactured, distributed, or labeled by Cosmuses Cosmetics (Ningbo) Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ANTIBACTERIAL HAND SANITIZER 01- alcohol liquid
Cosmuses Cosmetics (Ningbo) Co., Ltd.
----------
antibacterial hand sanitizer
When using this product, keep out of eyes. In case of contact with eyes, flush thoroughly with water. Avoid contact with broken skin. Do not inhale or ingest.
Keep out of reach of children. If swallowed, get medical help or call a Poison Control Center right away.
Directions
- Wet hands throughly with product and allow to dry without wiping.
- For children under 6 age use only under adult supervision.
- Not recommended for infants.
| ANTIBACTERIAL HAND SANITIZER
01
alcohol liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Cosmuses Cosmetics (Ningbo) Co., Ltd. (725290934) |
| Registrant - Cosmuses Cosmetics (Ningbo) Co., Ltd. (725290934) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cosmuses Cosmetics (Ningbo) Co., Ltd. | 725290934 | manufacture(82953-014) | |
Revised: 6/2024
Document Id: 1c26aedd-8c6a-382c-e063-6394a90adabc
Set id: 19a43c79-0b30-7c00-e063-6394a90acdd8
Version: 2
Effective Time: 20240630