Paulas Choice Clear Daily Skin Clearing Treatment 2.5% Benzoyl Peroxide Extra Strength
Paulas Choice Clear Daily Skin Clearing Treatment by
Drug Labeling and Warnings
Paulas Choice Clear Daily Skin Clearing Treatment by is a Otc medication manufactured, distributed, or labeled by Paula's Choice, LLC, Thibiant International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
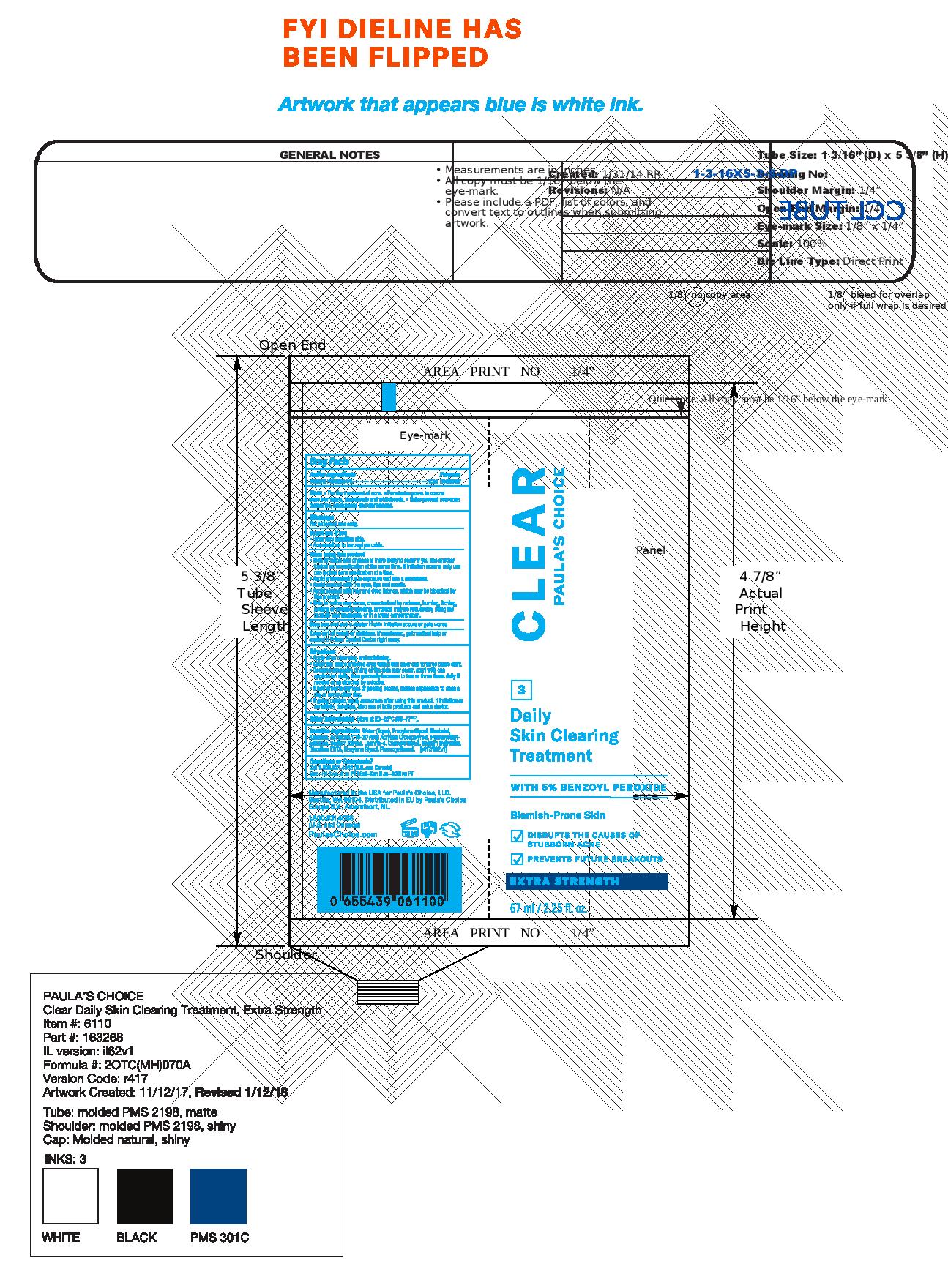
PAULAS CHOICE CLEAR DAILY SKIN CLEARING TREATMENT- benzoyl peroxide lotion
Paula's Choice, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Paulas Choice Clear Daily Skin Clearing Treatment 2.5% Benzoyl Peroxide Extra Strength
After cleansing with Paula's Choice CLEAR Pore Normalizing Cleanser and exfoliating with Anti-Redness Exfoliating Solution, cover the affected area with a thin layer 1 to 3 times a day. Because excessive drying of the skin may occur, start with 1 appliation daily and then gradually increase to 2 or 3 times daily if needed or as directed by a doctor. If bothersome dryness or peeling occurs, reduce application to once a day or every other day. If going outside, use a sunscreen. If sensitivity develops, discontinue use.
Do not use · on broken skin · on large areas of the body · if you are sensitive to benzoyl peroxide.
· Avoid unnecessary sun exposure and use a sunscreen · Avoid contact with eyes, lips and mouthIf contact occure, rinse with water · This product may bleach hair or dyed fabrics · Using other topical acne products at the sam etime or right after use of this product may increase dryness or irritation of the skin. If this occurs, only one drug should be used unless direct by a doctor.
Store at 20-26ºC (68-77ºF). · You may report serious adverse reactions to 1030 SW 34th Street, Suite A, Renton, WA 98057.
| PAULAS CHOICE CLEAR DAILY SKIN CLEARING TREATMENT
benzoyl peroxide lotion |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Paula's Choice, LLC (029583981) |
| Registrant - Paula's Choice, LLC (029583981) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Thibiant International, Inc. | 083913913 | manufacture(76144-611) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.