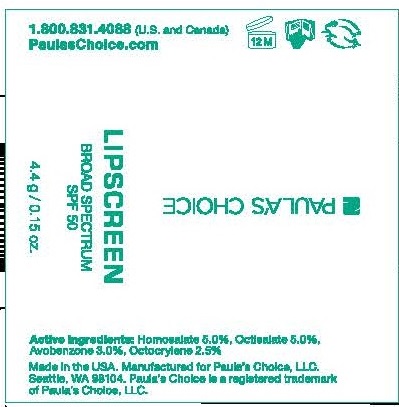
Paula's Choice Lipscreen SPF 50
Paulas Choice Lipscreen SPF 50 by
Drug Labeling and Warnings
Paulas Choice Lipscreen SPF 50 by is a Otc medication manufactured, distributed, or labeled by Paula's Choice, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PAULAS CHOICE LIPSCREEN SPF 50- homosalate, octisalate, avobenzone, octocrylene lipstick
Paula's Choice, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Paula's Choice Lipscreen SPF 50
Helps prevent sunburn. Decreases the risk of skin cancer and early skin aging caused by the sun if used as directed with other sun protection measures.
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center immediately.
Hydrogenated Soybean Oil, Bis-Diglyceryl Poluacyladipate-2, Hydrogenated Olive Oil, Polyglyceryl-3 Diisostearate, Beeswax, Ozokerite, Hydrogenated Jojoba Oil, Polyethylene, Hydrogenated Polycyclopentadiena, Microcrystalline Wax, Theobrome Cacao (Cocoa) Seed Butter, Silica, Tocopheryl Acetate, Phytosterols, Olea Europaea (Olive) Fruite Oil, Hydrogenated Vegetable Oil, Copernicia Cerifera (Caranauba) Wax, Water (Aqua), Phenoxyethanol.
| PAULAS CHOICE LIPSCREEN SPF 50
homosalate, octisalate, avobenzone, octocrylene lipstick |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Paula's Choice, LLC (029583981) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.