NALOXONE HYDROCHLORIDE spray
NALOXONE HYDROCHLORIDE by
Drug Labeling and Warnings
NALOXONE HYDROCHLORIDE by is a Otc medication manufactured, distributed, or labeled by HF Acquisition Co LLC, DBA HealthFirst. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT (IN EACH SPRAY)
- PURPOSE
- USES
- DIRECTIONS
- WARNINGS
- OTHER INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS?
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
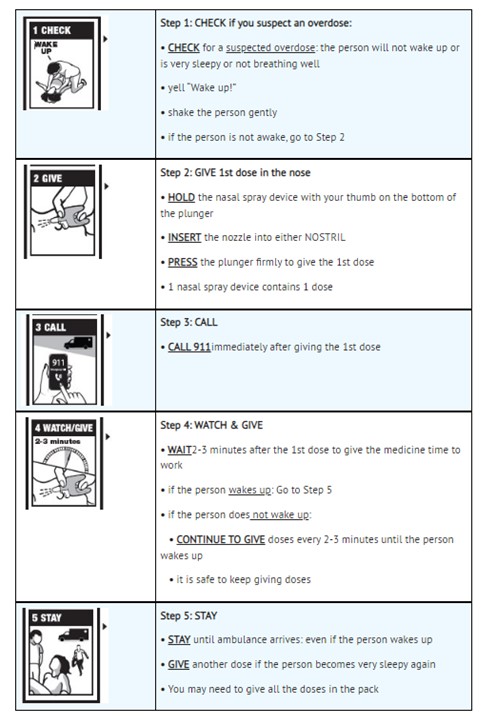
Step 1: CHECK if you suspect an overdose:
CHECK for a suspected overdose: the person will not wake up or is very sleepy or not breathing well
yell “Wake up!”
shake the person gently
if the person is not awake, go to Step 2
Step 2: GIVE 1st dose in the nose
HOLD the nasal spray device with your thumb on the bottom of the plunger
INSERT the nozzle into either NOSTRIL
PRESS the plunger firmly to give the 1st dose
1 nasal spray device contains 1 dose
Step 3: CALL
CALL 911immediately after giving the 1st dose
Step 4: WATCH & GIVE
WAIT2-3 minutes after the 1st dose to give the medicine time to work
if the person wakes up: Go to Step 5
if the person does not wake up:
CONTINUE TO GIVE doses every 2-3 minutes until the person wakes up
it is safe to keep giving doses
Step 5: STAY
STAY until ambulance arrives: even if the person wakes up
GIVE another dose if the person becomes very sleepy again
You may need to give all the doses in the pack
- INDICATIONS & USAGE
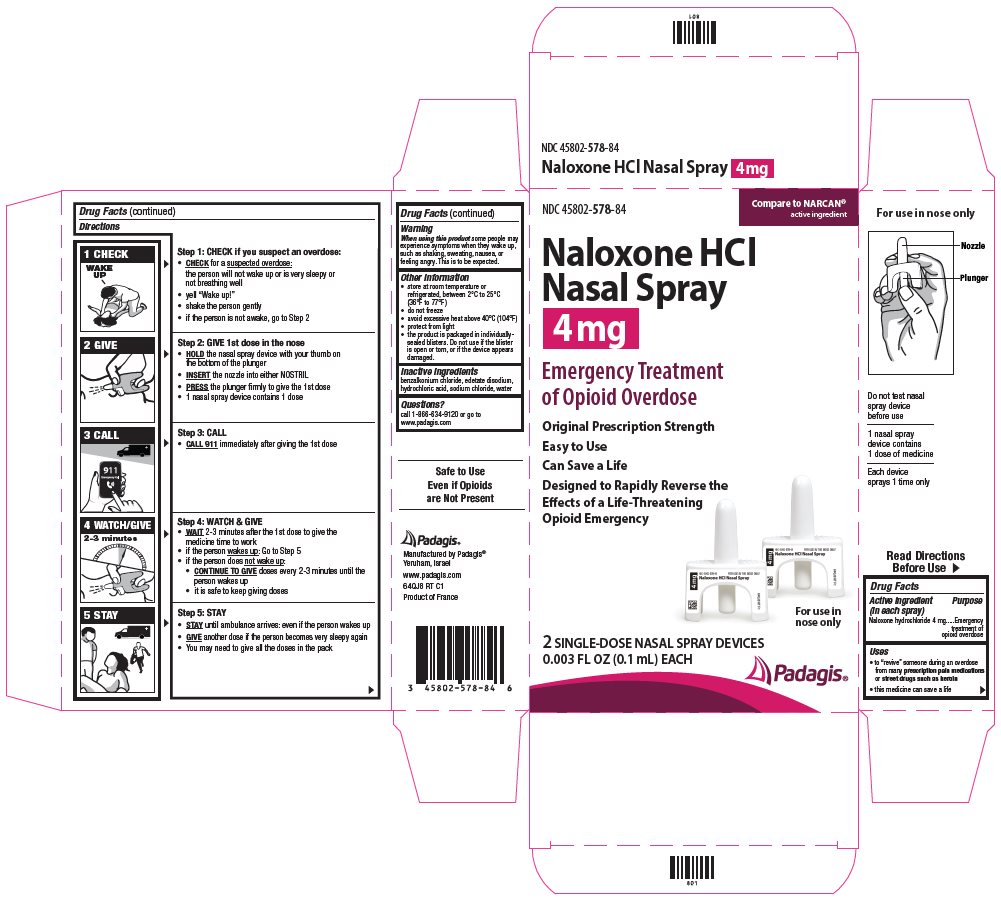
- NDC: 51662-1659-1
- NDC: 51662-1659-2
-
INGREDIENTS AND APPEARANCE
NALOXONE HYDROCHLORIDE
naloxone hydrochloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 51662-1659(NDC:45802-578) Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NALOXONE HYDROCHLORIDE (UNII: F850569PQR) (NALOXONE - UNII:36B82AMQ7N) NALOXONE HYDROCHLORIDE 4 mg in 0.1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51662-1659-2 2 in 1 CARTON 07/30/2023 1 NDC: 51662-1659-1 0.1 mL in 1 VIAL, SINGLE-DOSE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA211951 07/30/2023 Labeler - HF Acquisition Co LLC, DBA HealthFirst (045657305) Registrant - HF Acquisition Co LLC, DBA HealthFirst (045657305) Establishment Name Address ID/FEI Business Operations HF Acquisition Co LLC, DBA HealthFirst 045657305 relabel(51662-1659)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.