TOUCHLAND LIMITED EDITION POWER MIST LUSCIOUS FIVE SET- alcohol kit
Touchland Limited Edition Power Mist Luscious Five Set by
Drug Labeling and Warnings
Touchland Limited Edition Power Mist Luscious Five Set by is a Otc medication manufactured, distributed, or labeled by TOUCHLAND LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
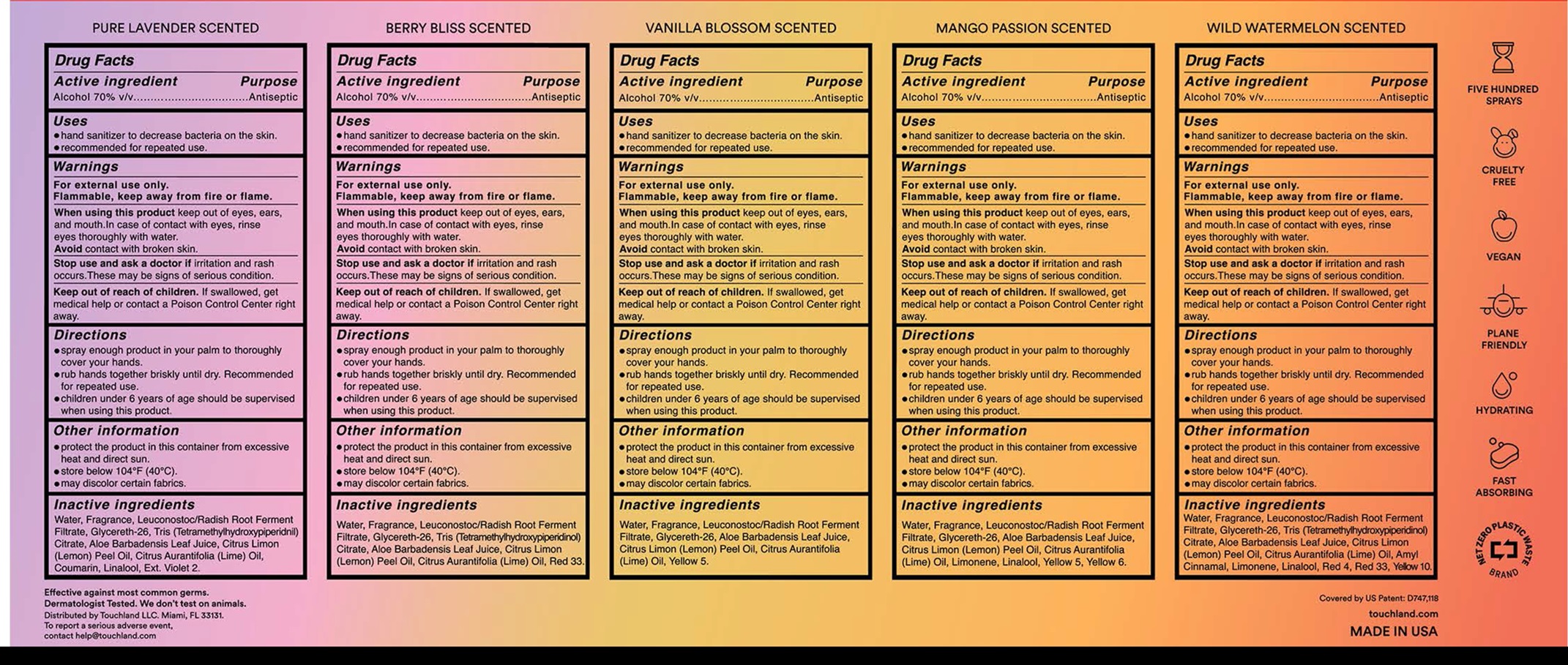
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
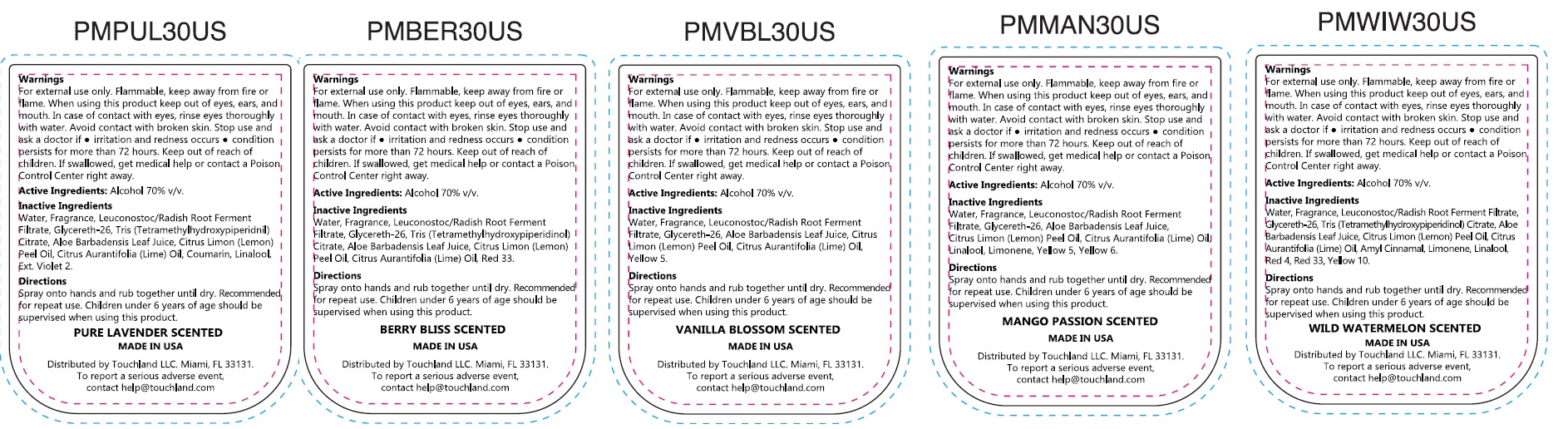
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
TOUCHLAND LIMITED EDITION POWER MIST LUSCIOUS FIVE SET
alcohol kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 72033-133 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72033-133-00 1 in 1 KIT 08/01/2024 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE 30 mL Part 2 1 BOTTLE 30 mL Part 3 1 BOTTLE 30 mL Part 4 1 BOTTLE 30 mL Part 5 1 BOTTLE 30 mL Part 1 of 5 TOUCHLAND POWER MIST PURE LAVANDER SCENTED HYDRATING HAND SANITIZER
alcohol sprayProduct Information Item Code (Source) NDC: 72033-134 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) GLYCERETH-26 (UNII: NNE56F2N14) ALOE VERA LEAF (UNII: ZY81Z83H0X) LEMON OIL, COLD PRESSED (UNII: I9GRO824LL) LIME OIL, COLD PRESSED (UNII: UZH29XGA8G) COUMARIN (UNII: A4VZ22K1WT) LINALOOL, (+/-)- (UNII: D81QY6I88E) EXT. D&C VIOLET NO. 2 (UNII: G5UX3K0728) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72033-134-00 1 in 1 CARTON 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/01/2024 Part 2 of 5 TOUCHLAND POWER MIST BERRY BLISS SCENTED HYDRATING HAND SANITIZER
alcohol sprayProduct Information Item Code (Source) NDC: 72033-135 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) GLYCERETH-26 (UNII: NNE56F2N14) TRIS(TETRAMETHYLHYDROXYPIPERIDINOL) CITRATE (UNII: 7NW772I64Y) ALOE VERA LEAF (UNII: ZY81Z83H0X) LEMON OIL, COLD PRESSED (UNII: I9GRO824LL) LIME OIL, COLD PRESSED (UNII: UZH29XGA8G) D&C RED NO. 33 (UNII: 9DBA0SBB0L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72033-135-00 1 in 1 CARTON 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/01/2024 Part 3 of 5 TOUCHLAND POWER MIST VANILLA BLOOSOM SCENTED HYDRATING HAND SANITIZER
alcohol sprayProduct Information Item Code (Source) NDC: 72033-136 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) GLYCERETH-26 (UNII: NNE56F2N14) ALOE VERA LEAF (UNII: ZY81Z83H0X) LEMON OIL, COLD PRESSED (UNII: I9GRO824LL) LIME OIL, COLD PRESSED (UNII: UZH29XGA8G) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72033-136-00 1 in 1 CARTON 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/01/2024 Part 4 of 5 TOUCHLAND POWER MIST MANGO PASSION SCENTED HYDRATING HAND SANITIZER
alcohol sprayProduct Information Item Code (Source) NDC: 72033-137 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) GLYCERETH-26 (UNII: NNE56F2N14) ALOE VERA LEAF (UNII: ZY81Z83H0X) LEMON OIL, COLD PRESSED (UNII: I9GRO824LL) LIME OIL, COLD PRESSED (UNII: UZH29XGA8G) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72033-137-00 1 in 1 CARTON 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/01/2024 Part 5 of 5 TOUCHLAND POWER MIST WILD WATERMELON SCENTED HYDRATING HAND SANITIZER
alcohol sprayProduct Information Item Code (Source) NDC: 72033-138 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) GLYCERETH-26 (UNII: NNE56F2N14) TRIS(TETRAMETHYLHYDROXYPIPERIDINOL) CITRATE (UNII: 7NW772I64Y) ALOE VERA LEAF (UNII: ZY81Z83H0X) LEMON OIL, COLD PRESSED (UNII: I9GRO824LL) LIME OIL, COLD PRESSED (UNII: UZH29XGA8G) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) FD&C RED NO. 4 (UNII: X3W0AM1JLX) D&C RED NO. 33 (UNII: 9DBA0SBB0L) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72033-138-00 1 in 1 CARTON 1 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/01/2024 Labeler - TOUCHLAND LLC (036656461)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.