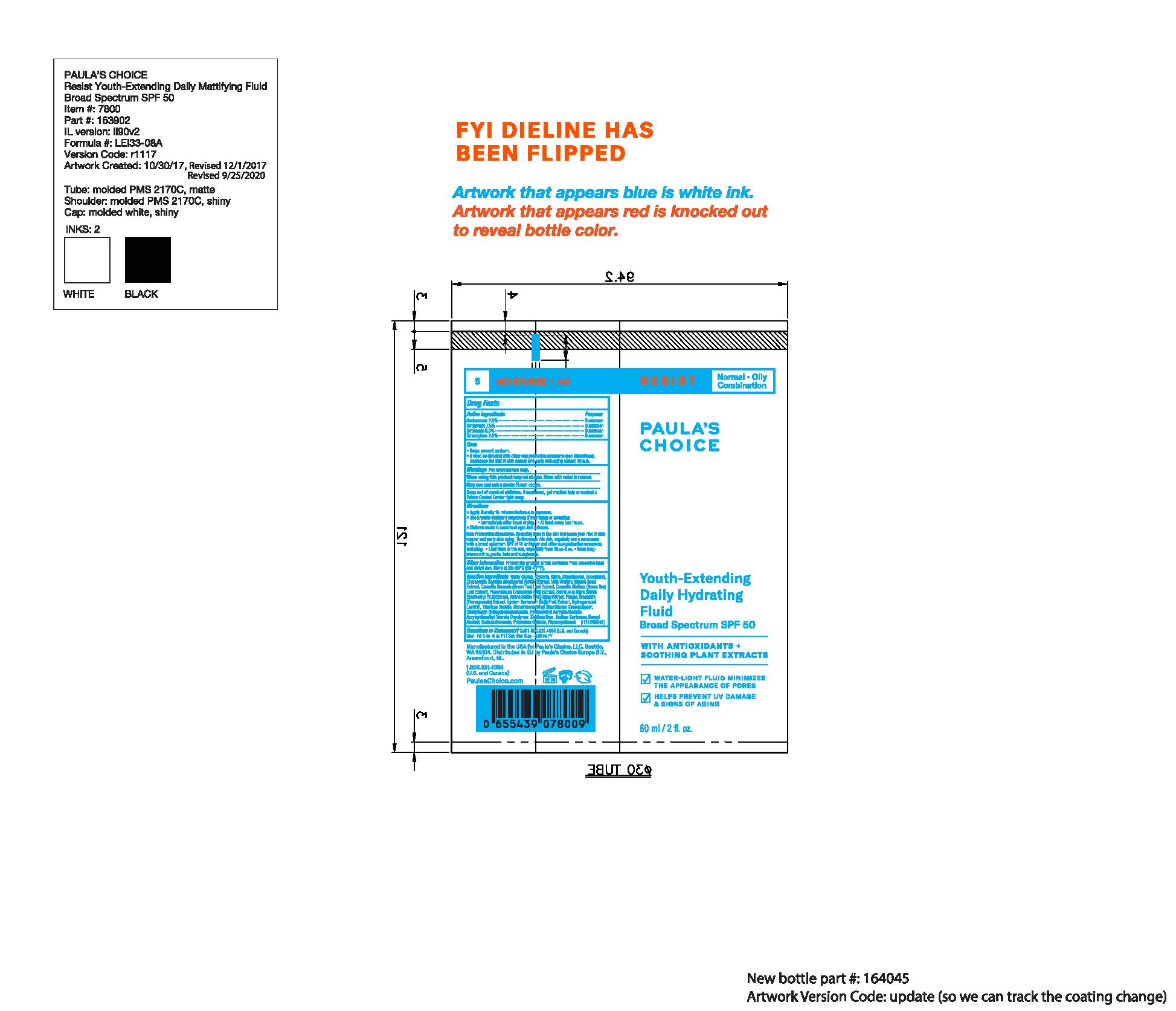
Paula's Choice Resist Youth-Extending Daily Hydrating Fluid SPF 50
Paulas Choice Resist Youth Extending Daily Hydrating Fluid SPF 50 by
Drug Labeling and Warnings
Paulas Choice Resist Youth Extending Daily Hydrating Fluid SPF 50 by is a Otc medication manufactured, distributed, or labeled by Paula's Choice, LLC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PAULAS CHOICE RESIST YOUTH EXTENDING DAILY HYDRATING FLUID SPF 50- avonbenzone, octinoxate, octisalate, octocrylene liquid
Paula's Choice, LLC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Paula's Choice Resist Youth-Extending Daily Hydrating Fluid SPF 50
Keep out of reach of children. If product is swalllowed, get medical help or contact a Poison Control Center immediately.
Directions
Apply liberally 15 minutes before sun exposure. Use a water-resistant sunscreen if swimming or sweating. Reapply at least every two hours if skin is exposed to sun.
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a broad-spectrum sunscreen rated SPF of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeve shirts, pants, hats, and sunglasses
- Children under 6 months of age: ask a doctor
Protect this product from excessive heat and direct sun. You may report serious adverse reactions to 705 5th Avenue South, Suite 200, Seattle, WA 98104
Water, Glycerin, Silica, Dimethicone, Tocopherol, Chamomilla Recutita (Matricaria) Flower Extract, Vitis Vinifera (Grape) Seed Extract, Camellia Sinensis (Green Tea) Leaf Extract, Camellia Oleifera (Green Tea) Leaf Extract, Peucedanum Graveolens (Dill) Extract, Sambucus Nigra (Black Elderberry) Fruit Extract, Avena Sativa (Oat) Bran Extract, Punica Granatum (Pomegranate) Extract, Lycium Barbarum (Goji) Fruit Extract, Hydrogenated Lecithin, Titanium Dioxide, Dimethicone/Vinyl Dimethicone Crosspolymer, Diethylhexyl Syringylidenemalonate, Potassium Sorbate, Sodium Benzoate, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Xanthan Gum, Sodium Carbomer, Benzyl Alcohol, Phenoxyethanol
| PAULAS CHOICE RESIST YOUTH EXTENDING DAILY HYDRATING FLUID SPF 50
avonbenzone, octinoxate, octisalate, octocrylene liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Paula's Choice, LLC. (029583981) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.