PAIN RELIEF PATCH- lidocaine 4% patch
pain relief patch by
Drug Labeling and Warnings
pain relief patch by is a Otc medication manufactured, distributed, or labeled by Bionpharma Inc., Sparsha Pharma USA, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only.
Do not use
■ If you are allergic to any active or inactive ingredients of this product ■ On wounds or damaged, irritated or sensitive skin
■ More than one patch on your body at a time or with other topical analgesics at the same time
■ For more than one week without consulting a doctor ■ With a heating pad ■ If pouch is damaged or opened.When using this product
■ Avoid contact with the eyes ■ Use only as directed ■ Read and follow all directions and warnings on this
package ■ Do not bandage tightly or apply local heat to the area of use ■ Do not reuse patch ■ Keep this product away from pets and
children. Used patches still contain the drug product that can produce serious adverse effect if a child or pet chews or ingests this
patch -
Directions
■ Adults and children 12 years of age and over: Clean and dry affected area ■ Apply immediately upon removal from pouch ■ Peel off
the first liner and apply exposed patch to affected area ■ Peel off second liner while applying the rest of patch ■ Remove patch after
24 hours ■ Discard patch after single use.
■ Children under 12 years of age: Consult a doctor. - Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
-
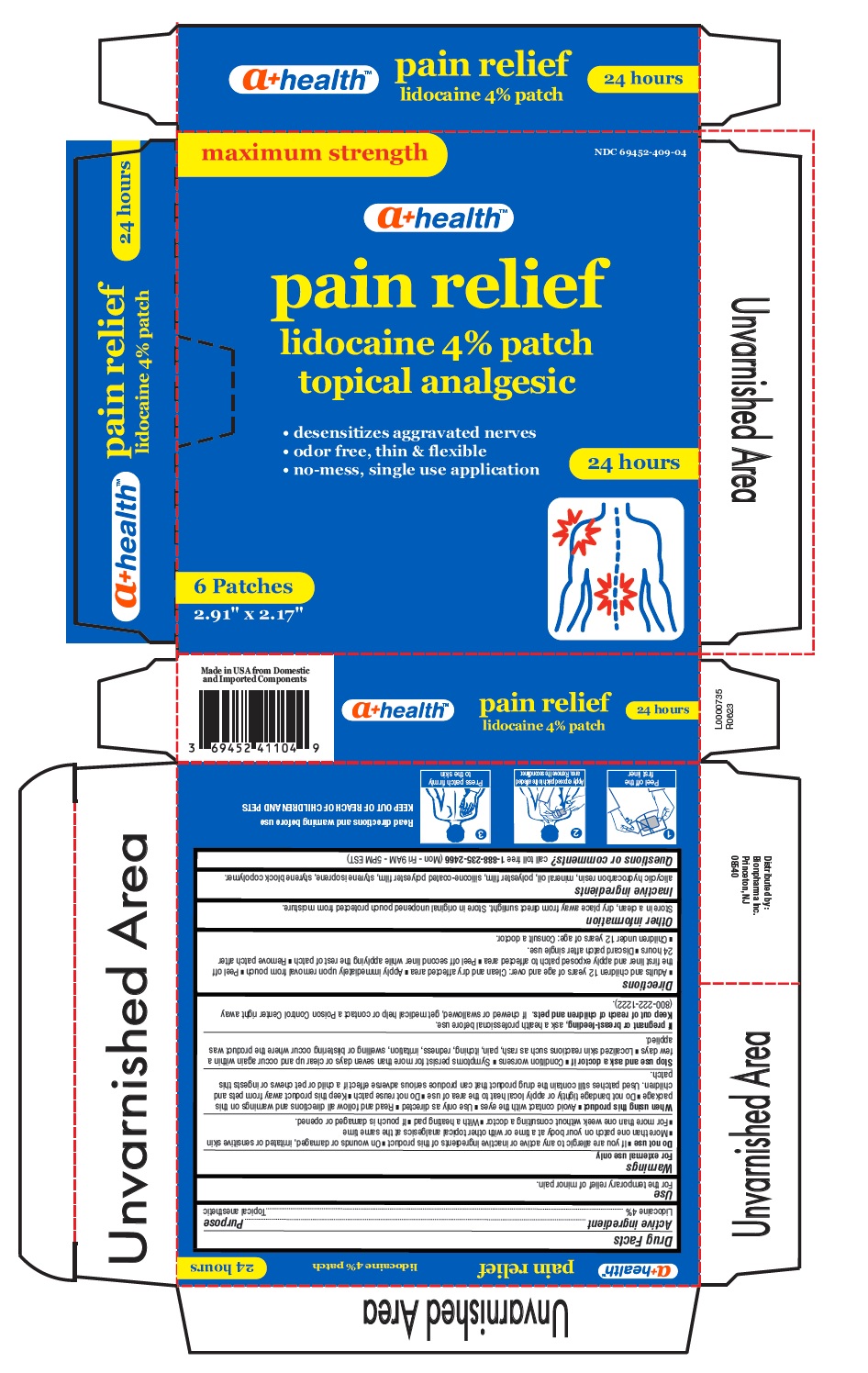
Carton Label
NDC: 69452-409-04
maximum strength
a+ health TM
pain relief
lidocaine 4% patch
topical analgesicdesensitizes aggravated nerves
odor free, thin & flexible
no-mess, single use application24 hours
6 Patches
2.91" x 2.17"
-
INGREDIENTS AND APPEARANCE
PAIN RELIEF PATCH
lidocaine 4% patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69452-409 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 g Inactive Ingredients Ingredient Name Strength HYDROGENATED C6-20 POLYOLEFIN (100 CST) (UNII: 39EYQ1W9RB) STYRENE/ISOPRENE/STYRENE BLOCK COPOLYMER (STYRENE/ISOPRENE 15/85) (UNII: 1SSZ6HXE7P) MINERAL OIL (UNII: T5L8T28FGP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69452-409-04 6 in 1 CARTON 10/04/2024 1 1 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 10/04/2024 Labeler - Bionpharma Inc. (079637826) Registrant - Bionpharma Inc. (079637826) Establishment Name Address ID/FEI Business Operations Sparsha Pharma USA, Inc. 079365856 manufacture(69452-409)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.