NEXOBRID- anacaulase-bcdb kit
NEXOBRID by
Drug Labeling and Warnings
NEXOBRID by is a Prescription medication manufactured, distributed, or labeled by Vericel Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NEXOBRID safely and effectively. See full prescribing information for NEXOBRID.
NEXOBRID® (anacaulase-bcdb), for topical gel
Initial U.S. Approval: 2022INDICATIONS AND USAGE
NEXOBRID contains proteolytic enzymes and is indicated for eschar removal in adults and pediatric patients with deep partial thickness and/or full thickness thermal burns (1).
Limitations of Use
The safety and effectiveness of NEXOBRID have not been established for treatment of:
- Chemical or electrical burns (1).
- Burns on the face, perineum, or genitalia (1).
- Burns on the feet of patients with diabetes mellitus or on the feet of patients with occlusive vascular disease (1).
- Circumferential burns (1).
- Burns in patients with significant cardiopulmonary disease, including inhalation injury (1).
NEXOBRID is not recommended for:
- Wounds contaminated with radioactive and other hazardous substances to avoid unforeseeable reactions with the product and an increased risk of spreading the noxious substance (1)
- Treatment of burn wounds where medical devices (e.g., implants, pacemakers, shunts) or vital structures (e.g., large vessels) could become exposed during eschar removal (1).
DOSAGE AND ADMINISTRATION
- NEXOBRID lyophilized powder and gel vehicle must be mixed prior to administration (2.1).
- Use 2 g of Nexobrid lyophilized powder mixed with 20 g gel for treatment of up to 180 cm2 of treated burn area; or 5 g of Nexobrid lyophilized powder mixed with 50 g gel for treatment of up to 450 cm2 of treated burn area (2.1).
- For topical use only (2.1).
- Dosage in Adults:
Apply a 3 mm thick layer of NEXOBRID in one application to an area of up to 15% body surface area (BSA) for four hours. A second application may be applied 24 hours later. For both applications, the total treated area must not exceed 20% BSA (2.2).
- Dosage in Pediatric Patients 6 Years of Age and Older:
Apply a 3 mm thick layer of NEXOBRID in one application of 4 hours to an area of up to 15% BSA (2.2).
- Dosage in Pediatric Patients Less Than 6 Years of Age:
Apply a 3 mm thick layer of NEXOBRID in one application of 4 hours to an area of up to 10% BSA (2.2).
- Prepare NEXOBRID at patient’s bedside within 15 minutes of intended application (2.4).
- Apply NEXOBRID to a clean, moist wound bed free of burned epidermis layer and blisters, and cover with an occlusive film dressing (2.3, 2.4).
- See Full Prescribing Information for additional details on preparation, administration, and removal of NEXOBRID (2.5, 2.6).
DOSAGE FORMS AND STRENGTHS
- For topical gel: 8.8% (3).
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hypersensitivity reactions: Serious hypersensitivity reactions, including anaphylaxis, have been reported with postmarketing use of anacaulase-bcdb. If a hypersensitivity reaction occurs, remove NEXOBRID (if applicable) and initiate appropriate therapy (5.1).
- Coagulopathy: Avoid use of NEXOBRID in patients with uncontrolled disorders of coagulation. Use with caution in patients on anticoagulant therapy or other drugs affecting coagulation, and in patients with low platelet counts and increased risk of bleeding from other causes. Monitor patients for possible signs of coagulation abnormalities and signs of bleeding (5.2).
ADVERSE REACTIONS
The most common adverse reactions (>10%) were pruritus and pyrexia (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Vericel Corporation at 1-888-454-BURN or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Information
2.2 Recommended Dosage
2.3 Preparation of Patient and Burn Wound Treatment Area
2.4 Preparation and Application of NEXOBRID
2.5 Removal of NEXOBRID
2.6 Monitoring
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Coagulopathy
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Adults
14.2 Pediatric Subjects
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
NEXOBRID is indicated for eschar removal in adults and pediatric patients with deep partial thickness (DPT) and/or full thickness (FT) thermal burns.
Limitations of Use
The safety and effectiveness of NEXOBRID have not been established for treatment of:
- Chemical or electrical burns
- Burns on the face, perineum, or genitalia
- Burns on the feet of patients with diabetes mellitus or on the feet of patients with occlusive vascular disease
- Circumferential burns
- Burns in patients with significant cardiopulmonary disease, including inhalation injury
NEXOBRID is not recommended for:
- Wounds contaminated with radioactive and other hazardous substances to avoid unforeseeable reactions with the product and an increased risk of spreading the noxious substance.
- Treatment of burn wounds where medical devices (e.g., implants, pacemakers, shunts) or vital structures (e.g., large vessels) could become exposed during eschar removal.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Information
NEXOBRID is only to be administered by a healthcare provider. Take precautions to avoid exposure to NEXOBRID during preparation and handling (e.g., use gloves, surgical masks, other protective coverings, as needed) [see Warnings and Precautions (5.1)].
Use pain management as practiced for an extensive dressing change of burn wounds 15 minutes prior to and throughout all NEXOBRID-related procedures.
NEXOBRID is available as:
- 2 g of lyophilized powder (containing 1.94 grams of anacaulase-bcdb) with a 20 g gel vehicle, for treatment of up to 180 cm2 of burn area after mixing.
- 5 g lyophilized powder (containing 4.85 grams of anacaulase-bcdb) with a 50 g gel vehicle, for treatment of up to 450 cm2 of burn area after mixing.
NEXOBRID lyophilized powder and gel vehicle must be mixed no more than 15 minutes prior to administration. Discard NEXOBRID if not used within 15 minutes of preparation, as the enzymatic activity of the product decreases progressively following mixing.
Each vial of lyophilized powder, jar of gel vehicle, and the mixed NEXOBRID are for use for only one patient and for one application [see Dosage and Administration (2.4)].
NEXOBRID is for topical use only.
Apply an ointment skin protectant around the treatment area to create an ointment barrier [see Dosage and Administration (2.3)].
2.2 Recommended Dosage
Recommended Dosage in Adults
Apply a 3 mm thick layer (approximate thickness of a tongue depressor) of NEXOBRID to a burn wound area of up to 15% body surface area (BSA) in one application in adult patients. Remove NEXOBRID after 4 hours [see Dosage and Administration (2.5)].
A second application of NEXOBRID may be applied 24 hours following the first application to either the same area previously treated with NEXOBRID or to a new area in adult patients. Apply a second application if:- The wound area is more than 15% BSA, or
- Multiple wound areas on different body surfaces require two treatments for logistical reasons such as body position, or
- The first application's eschar removal was not complete.
For both applications, the total treated area must not exceed 20% BSA.
Recommended Dosage in Pediatric Patients
Pediatric Patients 6 Years of Age and Older
Apply a 3 mm thick layer (approximate thickness of a tongue depressor) of NEXOBRID to a burn wound area of up to 15% BSA in one application in pediatric patients 6 years of age and older. Remove NEXOBRID after 4 hours [see Dosage and Administration (2.5)]. A second application of NEXOBRID is not recommended.
Pediatric Patients Less Than 6 Years of Age
Apply a 3 mm thick layer (approximate thickness of a tongue depressor) of NEXOBRID to a burn wound area of up to 10% BSA in one application in pediatric patients less than 6 years of age. Remove NEXOBRID after 4 hours [see Dosage and Administration (2.5)]. A second application of NEXOBRID is not recommended.
2.3 Preparation of Patient and Burn Wound Treatment Area
Prepare the wound area as follows:
- Thoroughly clean the wound to remove any charred tissue, blisters, and any topical products.
- Apply a dressing soaked with an antibacterial solution to the treatment area for at least 2 hours.
- Ensure the wound bed is clear of any remnants of topical agents (e.g., silver sulfadiazine, povidone iodine).
- Apply an ointment skin protectant (e.g., petrolatum) 2 to 3 cm outside of the treatment area to create an ointment barrier. Avoid applying the protectant ointment to the treatment area itself, as this would impede direct contact of NEXOBRID with the eschar.
- Protect any other open wounds (e.g., laceration, abraded skin, escharotomy incision) with skin protectant ointments or ointment gauze to prevent possible exposure to NEXOBRID.
2.4 Preparation and Application of NEXOBRID
Gather the following sterile supplies prior to NEXOBRID preparation and application:
- Instrument for mixing (e.g., spatula or tongue depressor)
- Tongue depressor for NEXOBRID application
- 0.9% Sodium Chloride Irrigation
- Occlusive film dressing
- Loose, thick fluffy dressing and bandage
Preparation
Prepare NEXOBRID at the patient’s bedside within 15 minutes of the intended application.
Using aseptic technique, mix NEXOBRID lyophilized powder and gel vehicle as follows:- Pour the NEXOBRID lyophilized powder into the gel vehicle jar.
- Thoroughly mix the NEXOBRID lyophilized powder and gel vehicle using a sterile instrument (e.g., tongue depressor or spatula) until the mixture is uniform. The mixed lyophilized powder and gel vehicle produce NEXOBRID in a final concentration of 8.8% w/w.
DISCARD NEXOBRID IF NOT USED WITHIN 15 MINUTES OF PREPARATION, as the enzymatic activity of NEXOBRID decreases progressively following mixing.
Application
Apply NEXOBRID within 15 minutes of preparation as follows:- Moisten the treatment area by sprinkling sterile 0.9% Sodium Chloride Irrigation onto the burn wound.
- Using a sterile tongue depressor, completely cover the moistened burn wound treatment area with the mixed NEXOBRID in a 3 mm thick layer (approximate thickness of a tongue depressor). Ensure NEXOBRID covers the entire target treatment area.
- Cover the treated wound with a sterile occlusive film dressing.
- Gently press the occlusive film dressing at the area of contact with the ointment barrier to ensure adherence between the occlusive film dressing and the ointment barrier and to achieve complete containment of NEXOBRID on the treatment area. There should be no visible air under the occlusive film dressing.
- Cover the occlusive film dressing with a sterile loose, thick, fluffy dressing and secure with a sterile bandage.
- Discard any unused portions of NEXOBRID.
2.5 Removal of NEXOBRID
Remove NEXOBRID after 4 hours. Gather the following sterile supplies prior to NEXOBRID removal:
- Blunt-edged instruments (e.g., tongue depressor)
- Large dry gauze
- Gauze soaked with 0.9% Sodium Chloride Irrigation
- Dressing soaked with an antibacterial solution
- Remove the occlusive film dressing using aseptic technique.
- Remove the ointment barrier using a sterile blunt-edged instrument.
- Remove the dissolved eschar from the wound by scraping it away with a sterile blunt-edged instrument.
- Wipe the wound thoroughly with a large sterile dry gauze, then wipe with a sterile gauze that has been soaked with sterile 0.9% Sodium Chloride Irrigation. Rub the treated area until the appearance of a clean dermis or subcutaneous tissues with pinpoint bleeding.
- To remove remnants of dissolved eschar, apply a dressing soaked with an antibacterial solution for at least 2 hours.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
NEXOBRID-Treated Patients
Serious hypersensitivity reactions, including anaphylaxis, have been reported with postmarketing use of NEXOBRID. If a hypersensitivity reaction occurs, remove NEXOBRID (if applicable) and initiate appropriate therapy.
NEXOBRID is contraindicated in patients with a known hypersensitivity to anacaulase- bcdb, bromelain, pineapples or to any other component of NEXOBRID. NEXOBRID is also contraindicated in patients with known hypersensitivity to papayas or papain because of the risk of cross-sensitivity.
Healthcare Providers Preparing and Applying NEXOBRID
Healthcare personnel should take appropriate precautions to avoid exposure when preparing and handling NEXOBRID (e.g., gloves, surgical masks, other protective coverings, as needed). In the event of inadvertent skin exposure, rinse NEXOBRID off with water to reduce the likelihood of skin sensitization.
5.2 Coagulopathy
A reduction of platelet aggregation and plasma fibrinogen levels and a moderate increase in partial thromboplastin and prothrombin times have been reported in the literature as possible effects following oral administration of bromelain, a component of NEXOBRID. In vitro and animal data suggest that bromelain can also promote fibrinolysis.
Avoid use of NEXOBRID in patients with uncontrolled disorders of coagulation. Use NEXOBRID with caution in patients on anticoagulant therapy or other drugs affecting coagulation, and in patients with low platelet counts and increased risk of bleeding from other causes (e.g., peptic ulcers and sepsis). Monitor patients for possible signs of coagulation abnormalities and signs of bleeding. -
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a product cannot be directly compared to rates in the clinical trials of another product and may not reflect the rates observed in practice.
Adults:
Studies 1 and 2 evaluated subjects undergoing eschar removal for deep partial thickness (DPT) and/or full thickness (FT) thermal burns [see Clinical Studies (14)]. An integrated analysis of safety data from Studies 1 and 2 compared NEXOBRID (n=177) to standard of care (SOC) (n=149). The SOC treatment included both surgical and non-surgical eschar removal methods.
The mean age of the safety population was 35.6 years; 73% were male; 81% were White, 9% were Black, 6% were other races, and 3% were Asian. Regarding burn etiology, 65% had fire/flame burns, 26% had scald burns, 8% had contact burns, and <1% had burns of other etiology. The mean body surface area (BSA) of wounds that received study treatment was 9.2±5.07%. Mean BSA of all burn wounds was 12.0±6.05%. Of the 177 subjects who were treated with NEXOBRID in Studies 1 and 2, 159 (90%) received one treatment of NEXOBRID, and 18 (10%) received 2 treatments.
Table 1 presents adverse reactions that occurred in ≥ 1% of subjects in the NEXOBRID arm and at a higher incidence than the SOC arm, up to 3 months following wound closure.
Table 1: Adverse Reactions Reported in ≥1% and Greater Incidence than Standard of Care in NEXOBRID-Treated Patients in Studies 1 and 2a a During the time period from baseline to 3 months post wound closure b Standard of Care treatment included both surgical and non-surgical eschar removal methods c Wound complication includes skin graft failure, graft loss, graft complication, and wound decomposition NEXOBRID
(N = 177)
Patients
n (%)Standard of Careb
(N = 149)
Patients
n (%)Pruritus 27 (15) 19 (13) Pyrexia 21 (12) 13 (9) Wound complicationc 15 (9) 9 (6.0) Anemia 11 (6) 8 (5) Vomiting 9 (5) 4 (3) Insomnia 8 (5) 6 (4) Urinary tract infection 7 (4) 1 (1) Tachycardia 5 (3) 0 Rash 6 (3) 0 Infection 4 (2) 2 (1) Sepsis 4 (2) 1 (1) Leukocytosis 3 (2) 1 (1) Hypotension 3 (2) 1 (1) Hepatic function abnormal 2 (1) 0 Drug hypersensitivity 2 (1) 0 Bacteremia 2 (1) 0 Scar 2 (1) 0 Subcutaneous hematoma 2 (1) 0 Decubitus ulcer 2 (1) 0 Pediatric Subjects:
Study 3 (CIDS study) evaluated pediatric subjects undergoing eschar removal for deep partial thickness (DPT) and/or full thickness (FT) thermal burns [see Clinical Studies (14)]. A total of 139 subjects (69 NEXOBRID, 70 SOC) were treated in the study. The SOC treatment included both surgical and non-surgical eschar removal methods.
The mean age was comparable in the NEXOBRID (5.89 years) and SOC (5.75 years) arms. The majority of subjects were male (59% NEXOBRID, 69% SOC). For race, 70% NEXOBRID and 69% SOC subjects were White, 25% NEXOBRID and 23% SOC subjects were Asian, 4% NEXOBRID and 3% SOC subjects were Black or African American, and 1% NEXOBRID and 4% SOC subjects were other races. For ethnicity, 4% NEXOBRID and 10% SOC subjects identified as Hispanic or Latino. The majority of burns in both treatment arms (NEXOBRID and SOC) were due to scald (68% and 67%). The majority of patients in both treatment groups (NEXOBRID and SOC) had 1 treated wound (71% and 80%, respectively).
Table 2 presents adverse reactions that occurred in ≥ 1% of subjects in the NEXOBRID arm and at a higher incidence than the SOC arm, up to 3 months following wound closure.Table 2: Adverse Reactions Reported in ≥1% and Greater Incidence than Standard of Care in NEXOBRID-Treated Pediatric Subjects in Study 3a a During the time period from baseline to 3 months post wound closure b Standard of Care treatment included both surgical and non-surgical eschar removal methods NEXOBRID
(N = 69)
Patients
n (%)Standard of Careb
(N = 70)
Patients
n (%)Pruritus 9 (13) 7 (10) Pyrexia 7 (10) 4 (6) Vomiting 5 (7) 3 (4) Nausea 3 (4) 2 (3) Constipation 3 (4) 1 (1) Nasopharyngitis 3 (4) 1 (1) Rash 2 (3) 0 Hemoglobin decreased 2 (3) 0 Rhinovirus 2 (3) 0 Ear Infection 2 (3) 0 6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of anacaulase-bcdb outside of the United States. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune system disorders: Hypersensitivity, including anaphylaxis and urticaria
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on NEXOBRID use in pregnant women to evaluate for a drug associated risk of major birth defects, miscarriage, or other adverse maternal or fetal outcomes.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Animal Data
In embryofetal developmental studies in rats and rabbits, intravenous doses up to 4 and 0.1 mg/kg/day NEXOBRID were administered to pregnant rats and rabbits, respectively, during organogenesis. No significant developmental toxicities were observed in these studies. However, severe maternal toxicities were noted and the tolerable maternal exposure levels were much lower compared with the maximum human exposure in clinical setting.
8.2 Lactation
Risk Summary
There are no data on the presence of anacaulase-bcdb in either human or animal milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for NEXOBRID and any potential adverse effects on the breastfed infant from NEXOBRID or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of NEXOBRID for eschar removal have been established in pediatric patients with deep partial thickness and/or full thickness thermal burns. Use of NEXOBRID for this indication is supported by evidence from one adequate and well-controlled trial in 69 pediatric subjects treated with NEXOBRID [see Clinical Studies (14)].
8.5 Geriatric Use
Of the 177 subjects exposed to NEXOBRID for eschar removal in deep partial thickness (DPT) and/or full thickness (FT) thermal burns, 6 (3%) subjects were 65 years or older, and 1 (< 1%) subject was 75 years or older [see Clinical Studies (14)]. Clinical trials of NEXOBRID did not include sufficient numbers of subjects 65 years of age and older to determine whether they respond differently from younger adult subjects.
-
11 DESCRIPTION
The drug substance in NEXOBRID, anacaulase-bcdb, is a mixture of proteolytic enzymes extracted from the stems of pineapple plants (Ananas comosus [L.] Merr.) that has been sterile filtered and lyophilized. The drug substance, anacaulase-bcdb, is composed mainly (80% to 95% w/w) of the proteins: stem bromelain, ananain, jacalin-like lectin, bromelain inhibitors, and phytocystatin inhibitor; and saccharides, as both free monosaccharides and the N-linked glycan of stem bromelain, and small molecule metabolites. The drug substance includes inactive buffer components containing acetic acid, ammonium sulfate, and Water for Injection. Each gram of lyophilized powder contains 0.97 grams of anacaulase-bcdb.
NEXOBRID (anacaulase-bcdb) for topical gel is a botanical drug product supplied as a sterile, preservative-free, lyophilized powder in a single-dose glass vial that must be mixed in a gel vehicle supplied in a single-dose glass jar prior to application. Mixture of either 2 grams of lyophilized powder (containing 1.94 grams of anacaulase-bcdb) or 5 grams of lyophilized powder (containing 4.85 grams of anacaulase-bcdb) in 20 grams or 50 grams of gel vehicle, respectively, provides an 8.8% w/w, yellowish white to light brown opaque gel for topical use. The pH of the topical gel mixture is approximately 6.2 to 6.7.
The co-packaged 20 gram or 50 gram jar of the sterile, preservative-free gel vehicle contains carbomer 980, dibasic sodium phosphate, sodium hydroxide, and Water for Injection.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mixture of enzymes in NEXOBRID dissolves burn wound eschar. The specific components responsible for this effect have not been identified.
12.2 Pharmacodynamics
The pharmacodynamics of NEXOBRID are unknown.
Cardiac Electrophysiology
At the approved recommended dose, NEXOBRID did not prolong the QT interval in humans to any clinically relevant extent.
12.3 Pharmacokinetics
Absorption
For adults and pediatric subjects 4 years of age and older, NEXOBRID applied to deep partial and full thickness burn wounds is absorbed, with median serum Tmax of 4 hours (during the treatment application). Systemic exposure (i.e., AUC) of bromelain, a component of anacaulase-bcdb is correlated with the size of the treated area and NEXOBRID dose, but not the depth of the burn wound. The systemic exposure in pediatric subjects was comparable to adults.
In adult subjects, Cmax and the dose‑normalized Cmax values after the first and second application (mean dosing interval of 17 hours) are comparable and only slight accumulation (less than 2-fold difference) is seen in AUC0-4 and AUC0-4 dose‑normalized levels after the second application, compared to the first application.Elimination
A majority of adults and pediatric subjects had no quantifiable serum concentrations after 72 hours. The mean ± SD terminal half-life of bromelain, a component of anacaulase-bcdb, is 12 ± 4.4 hours.
Drug Interaction Studies
Effect of NEXOBRID on Other Drugs
Bromelain, a component of anacaulase-bcdb, exhibited CYP2C8 time-dependent inhibition in human hepatocytes and inhibited human microsomal CYP2C9. No clinical studies have been conducted to assess the potential for systemic drug interactions.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Adults
The efficacy of NEXOBRID for the eschar removal of deep partial thickness (DPT) and full thickness (FT) thermal burns has been investigated in two trials.
Study 1
NEXOBRID was investigated in the DETECT randomized, controlled, assessor-blinded, three-arm trial, comparing NEXOBRID, standard of care (SOC), and gel vehicle treatment in subjects with DPT and/or FT thermal burns of 3 - 30% BSA (Study 1, NCT02148705). SOC included both surgical and non-surgical methods for eschar removal per the investigators’ discretion. Subjects on the NEXOBRID and gel vehicle arms who had eschar remaining following the topical treatment period were treated with SOC. NEXOBRID was compared to gel vehicle for the incidence of ≥95% eschar removal at the end of the topical treatment period. NEXOBRID was also compared with SOC for the incidence of surgical eschar removal (tangential, minor, avulsion, Versajet and/or dermabrasion excision) and time to eschar removal.
A total of 175 subjects were randomized in a 3:3:1 ratio (NEXOBRID : SOC : gel vehicle) and 169 subjects were treated. The mean age was 41 years, 70% of subjects were male and 30% were female, and 81% were White, 14% were Black or African American, 5% were other races, and 1% were Asian. Seventeen percent of subjects were Hispanic or Latino. Subjects had one or more target wounds (TWs) to be treated for eschar removal. The mean percentage BSA of all TWs per subject was 6.1%. The majority of subjects (82%) had one to two TWs.
The incidence of ≥95% eschar removal at the end of the topical treatment period for subjects in the NEXOBRID and gel vehicle groups is shown in Table 3.Table 3: Incidence of ≥95% Eschar Removal at the End of the Topical Treatment Period in NEXOBRID- or Gel Vehicle-Treated Subjects (Study 1; DETECT) NEXOBRID
(N=75)Gel Vehicle
(N=25)Treatment Difference
(95% Confidence Interval)93%
(70/75)4%
(1/25)89% (74%, 96%) The incidence of surgical eschar removal (tangential, minor, avulsion, Versajet and/or dermabrasion excision) and time to ≥95% eschar removal for the NEXOBRID and SOC groups are shown in Table 4.
Table 4: Incidence of Excision for Eschar Removal in NEXOBRID- or SOC-Treated Subjects (Study 1; DETECT) SOC = standard of care NEXOBRID
(N=75)SOC
(N=75)Treatment Difference
(95% Confidence Interval)4%
(3/75)72%
(54/75)-68% (-78%, -56%) The median time to eschar removal was 1 day on the NEXOBRID arm and 3.8 days on the SOC arm.
The estimated median time to ≥95% wound closure for all TWs on a subject was 31 days for the NEXOBRID arm and 36 days for the SOC treatment arm. Subjects were not evaluated frequently enough after achieving ≥95% wound closure to adequately assess time to 100% wound closure.
Study 2
NEXOBRID was investigated in a multicenter, open-label, randomized, two-arm trial, comparing NEXOBRID to SOC treatment in subjects with DPT and/or FT thermal burns of 5 - 24% BSA (Study 2; NCT00324311). SOC included both surgical and non-surgical methods for eschar removal per the investigators’ discretion. The trial enrolled 182 subjects. The first subject at each site (26 subjects) was not randomized and was treated with NEXOBRID. The remaining 156 subjects were randomized to NEXOBRID or SOC. The efficacy assessments were analyzed on DPT burns only.
Demographics were similar across both arms. The mean age was 29.9 years. Approximately 80% of the study subjects were adults (≥18 years), 74% were male and 26% were female, 82% were White, 7% were other races, 6% were Black, and 5% were Asian.
The incidence of surgical eschar removal (tangential, minor, avulsion, Versajet and/or dermabrasion excision) for the NEXOBRID and SOC groups is shown in Table 5.Table 5: Incidence of Excision of Eschar Removal of Deep Partial Thickness Wounds in Patients with Thermal Burns (Study 2) SOC = standard of care
aAnalysis population includes only patients with at least one wound that was entirely DPT
bSurgical eschar removal procedures include (tangential, minor, avulsion, Versajet and/or dermabrasion excision)
cAssessement per subject was an exploratory analysisNEXOBRID
N=106 Wounds
in 49 SubjectsaSOC
N=88 Wounds
in 48 SubjectsaTreatment Difference
(95% Confidence Interval)Incidence of excision for
eschar removal
(per wound)b15%
16/106 wounds63%
55/88 wounds-47% (-59%, -34%) Incidence of excision for
eschar removal
(per subject)b, c22%
11/49 subjects77%
37/48 subjects-55% (-71%, -38%) In randomized subjects, the estimated median time to ≥95% wound closure was 33 days for the NEXOBRID arm and 24 days for the SOC treatment arm. Subjects were not evaluated frequently enough after achieving ≥95% wound closure to adequately assess time to 100% wound closure.
14.2 Pediatric Subjects
Study 3
NEXOBRID was investigated in the CIDS study which was an open label, randomized, controlled, two arm trial comparing NEXOBRID and standard of care (SOC) treatment in subjects 7 months to 18 years of age with DPT and/or FT thermal burns of ≥1% - 30% BSA (Study 3, NCT02278718). SOC included both surgical and nonsurgical methods for eschar removal per the investigator’s discretion. Subjects on the NEXOBRID arm who had eschar remaining following the topical treatment period were treated with SOC. NEXOBRID was compared with SOC for the time to eschar removal and for the incidence of surgical eschar removal (tangential, minor, avulsion, Versajet and/or dermabrasion excision).
A total of 145 subjects were randomized in a 1:1 ratio (NEXOBRID:SOC) and 139 subjects were treated. The mean age was approximately 6 years, with a median of 3.4 years in the NEXOBRID group and 3.9 years in the SOC group. In the trial, 62% were male, 70% were White, 23% were Asian, 4% were Black or African American, and 3% were other races; for ethnicity, 7% identified as Hispanic or Latino. Subjects had one or more target wounds (TWs) to be treated for eschar removal. The mean percentage BSA of all TWs per subject was 5.6%. The majority of subjects in both treatment groups had 1 TW (71.0% for NEXOBRID and 80.0% for SOC).
The median time to ≥95% eschar removal is shown in Table 6.Table 6: Time to ≥95% Eschar Removal, Estimated Median Time for NEXOBRID vs SOC in Pediatric Subjects (Study 3; CIDS) NEXOBRID
(N=72)SOC
(N=73)Median (Days)
95% Confidence
Interval0.99
(0.88, 1.04)5.99
(2.71, 9.84)The incidence of surgical eschar removal (tangential, minor, avulsion, Versajet and/or dermabrasion excision) is shown in Table 7.
Table 7: Incidence of Surgical Excision for Eschar Removal for NEXOBRID vs SOC in Pediatric Subjects (Study 3; CIDS) NEXOBRID
(N=72)SOC
(N=73)Treatment Difference
(95% Confidence Interval)8%
(6/72)64%
(47/73)-56% (-68%, -42%) The estimated median time to ≥95% wound closure for all target wounds on a subject was 32 days for the NEXOBRID arm and 34 days for the SOC treatment arm. The estimated median time reach 100% wound closure on a target wound level was 44 days for NexoBrid arm and 43 days for the SOC arm.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
NEXOBRID (anacaulase-bcdb) for topical gel, 8.8%, is supplied as a package containing two components, a sterile, preservative-free, off-white to light tan lyophilized powder in a glass vial and a sterile, preservative-free, clear and colorless gel vehicle in a glass jar, that are mixed prior to application [see Dosage and Administration (2.4)].
NEXOBRID is available:
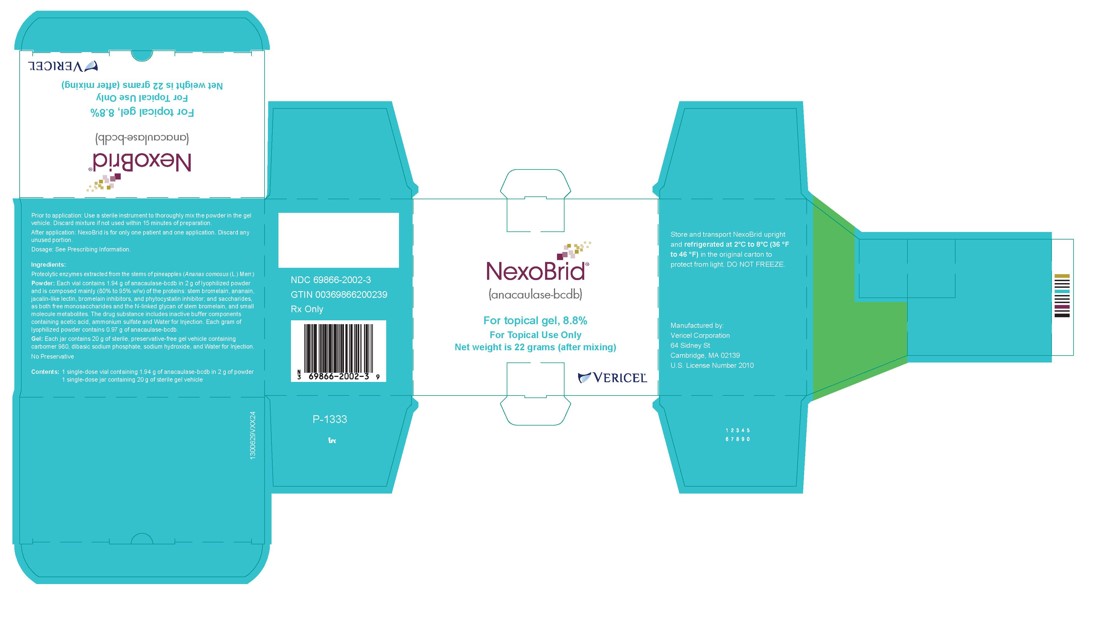
- One glass vial of 2 g lyophilized powder (containing 1.94 grams of anacaulase-bcdb) and one glass jar of 20 g gel vehicle per carton (NDC: 69866-2002-3)
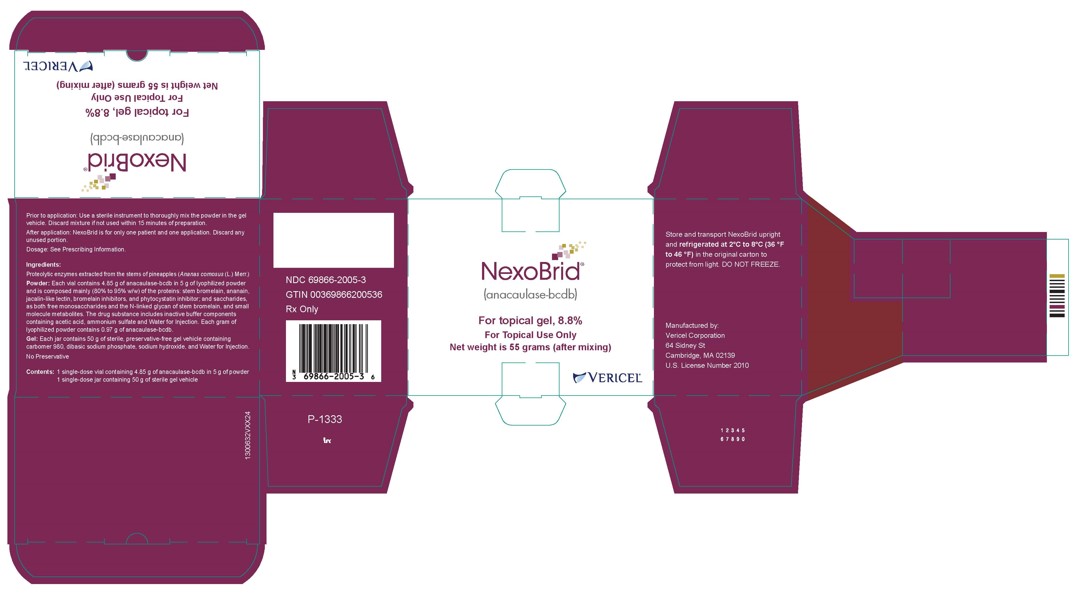
- One glass vial of 5 g lyophilized powder (containing 4.85 grams of anacaulase-bcdb) and one glass jar of 50 g gel vehicle per carton (NDC: 69866-2005-3)
Storage and Handling
Store and transport NEXOBRID package upright and refrigerated at 2℃ to 8℃ (36 ℉ to 46 ℉) in the original carton to protect from light. DO NOT FREEZE.
Do not use if the vial or jar are damaged.
-
17 PATIENT COUNSELING INFORMATION
Hypersensitivity Reactions
Advise patients of the risk of hypersensitivity reactions, including anaphylaxis [see Warnings and Precautions (5.1)].
Manufactured by:
Vericel Corporation
64 Sidney Street,
Cambridge, MA 02139
U.S. License number 2010
NEXOBRID® is a registered trademark of MediWound LTD.
L65629.6
- PRINCIPAL DISPLAY PANEL - NDC: 69866-2002-3 - 2g/20g Carton Label
- PRINCIPAL DISPLAY PANEL - NDC: 69866-2005-3 - 5g/50g Carton Label
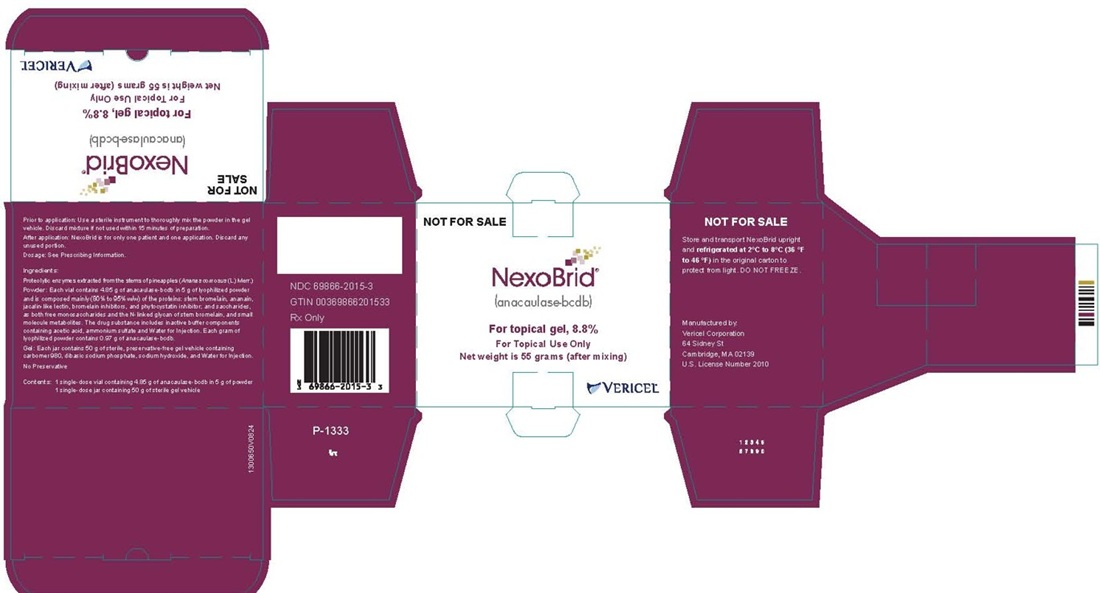
- PRINCIPAL DISPLAY PANEL - NDC: 69866-2015-3 - 5g/50g NFS Carton Label
-
INGREDIENTS AND APPEARANCE
NEXOBRID
anacaulase-bcdb kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69866-2002 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69866-2002-3 1 in 1 CARTON 12/29/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 2 g Part 2 1 JAR 20 g Part 1 of 2 NEXOBRID
anacaulase-bcdb powderProduct Information Item Code (Source) NDC: 69866-2000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANACAULASE-BCDB (UNII: JD24M53P9U) (ANACAULASE-BCDB - UNII:JD24M53P9U) ANACAULASE-BCDB 158 mg in 1 g Inactive Ingredients Ingredient Name Strength ACETIC ACID (UNII: Q40Q9N063P) AMMONIUM SULFATE (UNII: SU46BAM238) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69866-2000-1 2 g in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761192 12/29/2022 Part 2 of 2 STERILE
sterile gelProduct Information Item Code (Source) NDC: 69866-2001 Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69866-2001-2 20 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761192 12/29/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761192 12/29/2022 NEXOBRID
anacaulase-bcdb kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69866-2005 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69866-2005-3 1 in 1 CARTON 12/29/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 5 g Part 2 1 JAR 50 g Part 1 of 2 NEXOBRID
anacaulase-bcdb powderProduct Information Item Code (Source) NDC: 69866-2003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANACAULASE-BCDB (UNII: JD24M53P9U) (ANACAULASE-BCDB - UNII:JD24M53P9U) ANACAULASE-BCDB 158 mg in 1 g Inactive Ingredients Ingredient Name Strength ACETIC ACID (UNII: Q40Q9N063P) AMMONIUM SULFATE (UNII: SU46BAM238) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69866-2003-1 5 g in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761192 12/29/2022 Part 2 of 2 STERILE
sterile gelProduct Information Item Code (Source) NDC: 69866-2004 Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69866-2004-2 50 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761192 12/29/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761192 12/29/2022 NEXOBRID
anacaulase-bcdb kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69866-2015 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69866-2015-3 1 in 1 CARTON 12/29/2022 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL, GLASS 5 g Part 2 1 JAR 50 g Part 1 of 2 NEXOBRID
anacaulase-bcdb powderProduct Information Item Code (Source) NDC: 69866-2013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANACAULASE-BCDB (UNII: JD24M53P9U) (ANACAULASE-BCDB - UNII:JD24M53P9U) ANACAULASE-BCDB 158 mg in 1 g Inactive Ingredients Ingredient Name Strength ACETIC ACID (UNII: Q40Q9N063P) AMMONIUM SULFATE (UNII: SU46BAM238) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69866-2013-1 5 g in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761192 12/29/2022 Part 2 of 2 STERILE
sterile gelProduct Information Item Code (Source) NDC: 69866-2014 Route of Administration TOPICAL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69866-2014-2 50 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761192 12/29/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761192 12/29/2022 Labeler - Vericel Corporation (079745570)
Trademark Results [NEXOBRID]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NEXOBRID 79286826 not registered Live/Pending |
MediWound Ltd. 2020-05-04 |
 NEXOBRID 79089630 3964653 Live/Registered |
Mediwound Ltd 2010-10-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.