APOCREME- povidone iodine emulsion
Apocreme by
Drug Labeling and Warnings
Apocreme by is a Otc medication manufactured, distributed, or labeled by Microdermis Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
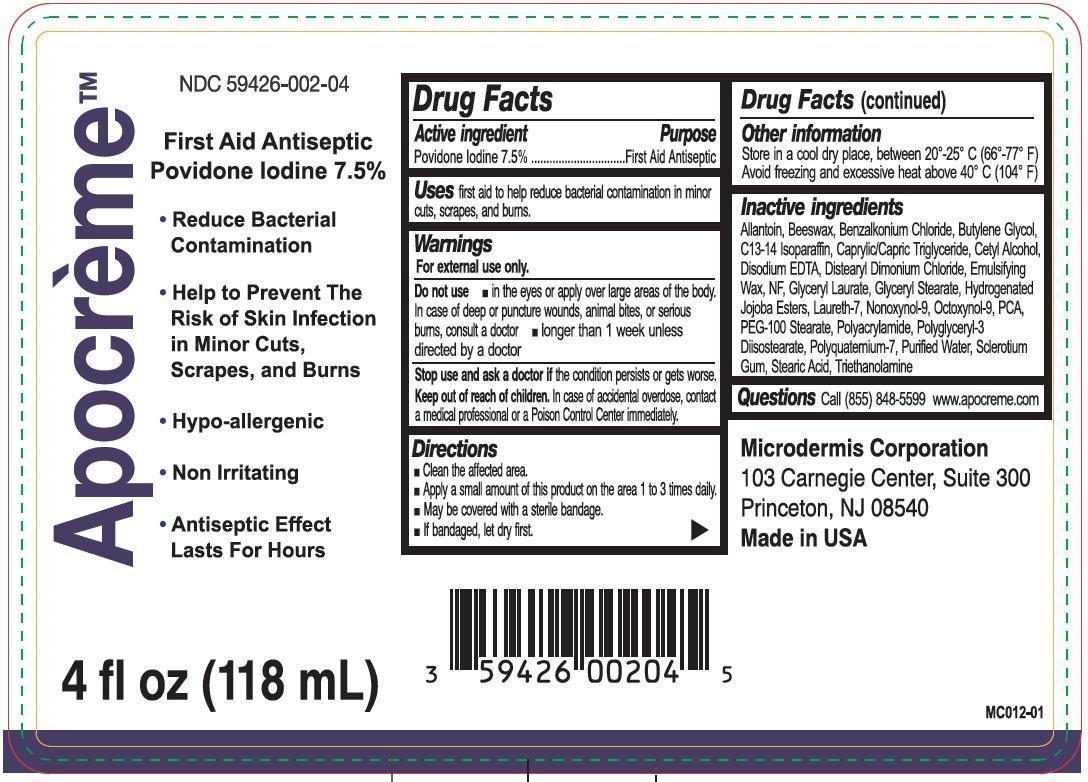
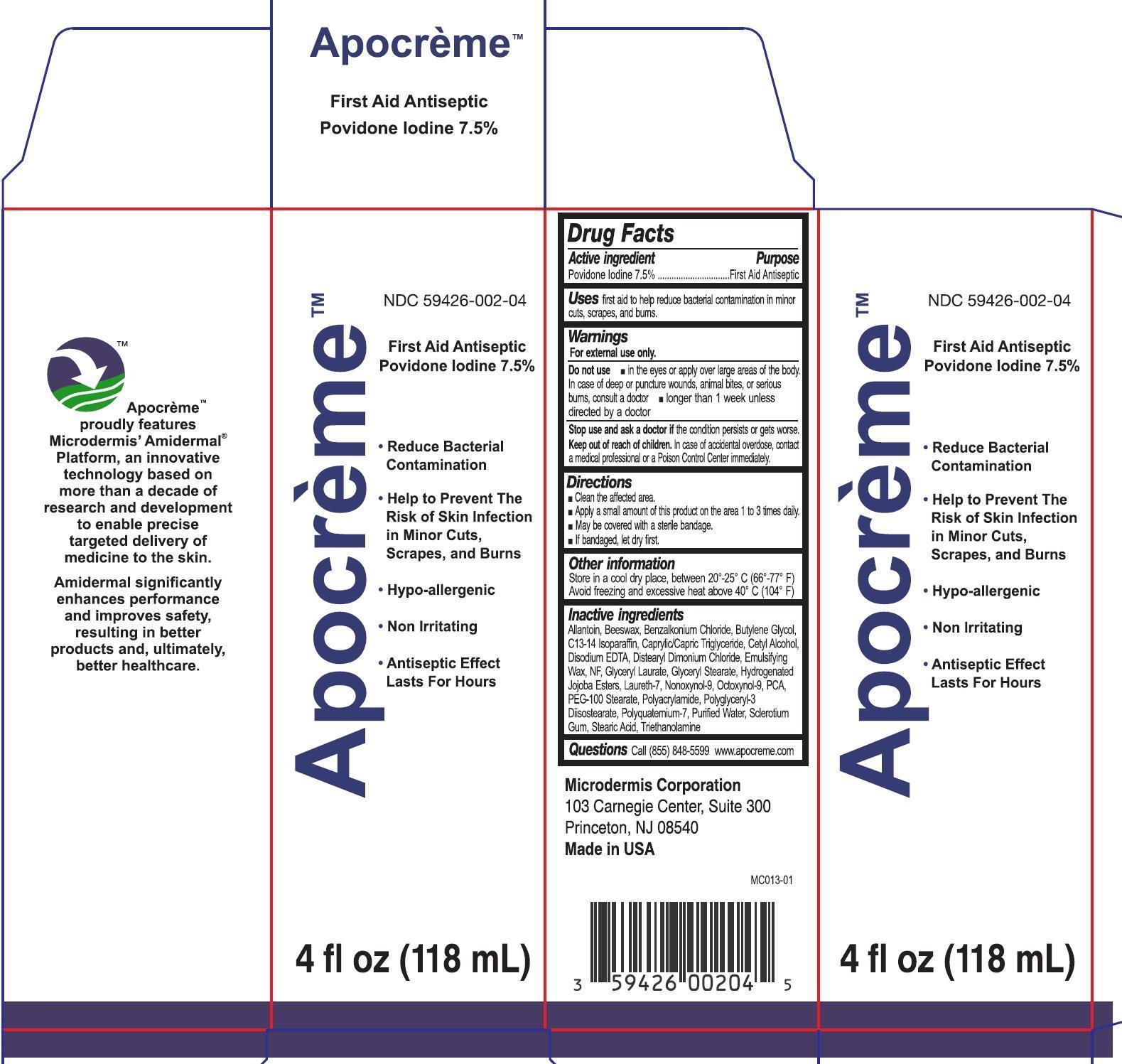
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
Allantoin, Beeswax, Benzalkonium Chloride, Butylene Glycol, C13-14 Isoparaffin, Caprylic/Capric Triglyceride, Cetyl Alcohol, Disodium EDTA, Distearyl Dimonium Chloride, Emulsifying Wax, NF, Glyceryl Laurate, Glyceryl Stearate, Hydeogenated Jojoba Esters, Laureth-7, Nonoxynol-9, Octoxynol-9, PCA, PEG-100 Stearate, Polyacrylamide, Polyglyceryl-3 Diisostearate, Polyquatemium-7, Purified Water, Sclerotium Gum, Stearic Acid, Triethanolamine
- Questions
- Product Label
-
INGREDIENTS AND APPEARANCE
APOCREME
povidone iodine emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59426-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 75 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALLANTOIN (UNII: 344S277G0Z) YELLOW WAX (UNII: 2ZA36H0S2V) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CETYL ALCOHOL (UNII: 936JST6JCN) EDETATE DISODIUM (UNII: 7FLD91C86K) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) GLYCERYL LAURATE (UNII: Y98611C087) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) LAURETH-7 (UNII: Z95S6G8201) NONOXYNOL-9 (UNII: 48Q180SH9T) OCTOXYNOL-9 (UNII: 7JPC6Y25QS) PIDOLIC ACID (UNII: SZB83O1W42) PEG-100 STEARATE (UNII: YD01N1999R) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) WATER (UNII: 059QF0KO0R) BETASIZOFIRAN (UNII: 2X51AD1X3T) STEARIC ACID (UNII: 4ELV7Z65AP) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59426-002-04 1 in 1 CARTON 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333C 06/21/2015 Labeler - Microdermis Corporation (969967988)
Trademark Results [Apocreme]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 APOCREME 86683619 not registered Dead/Abandoned |
Microdermis Corporation 2015-07-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.