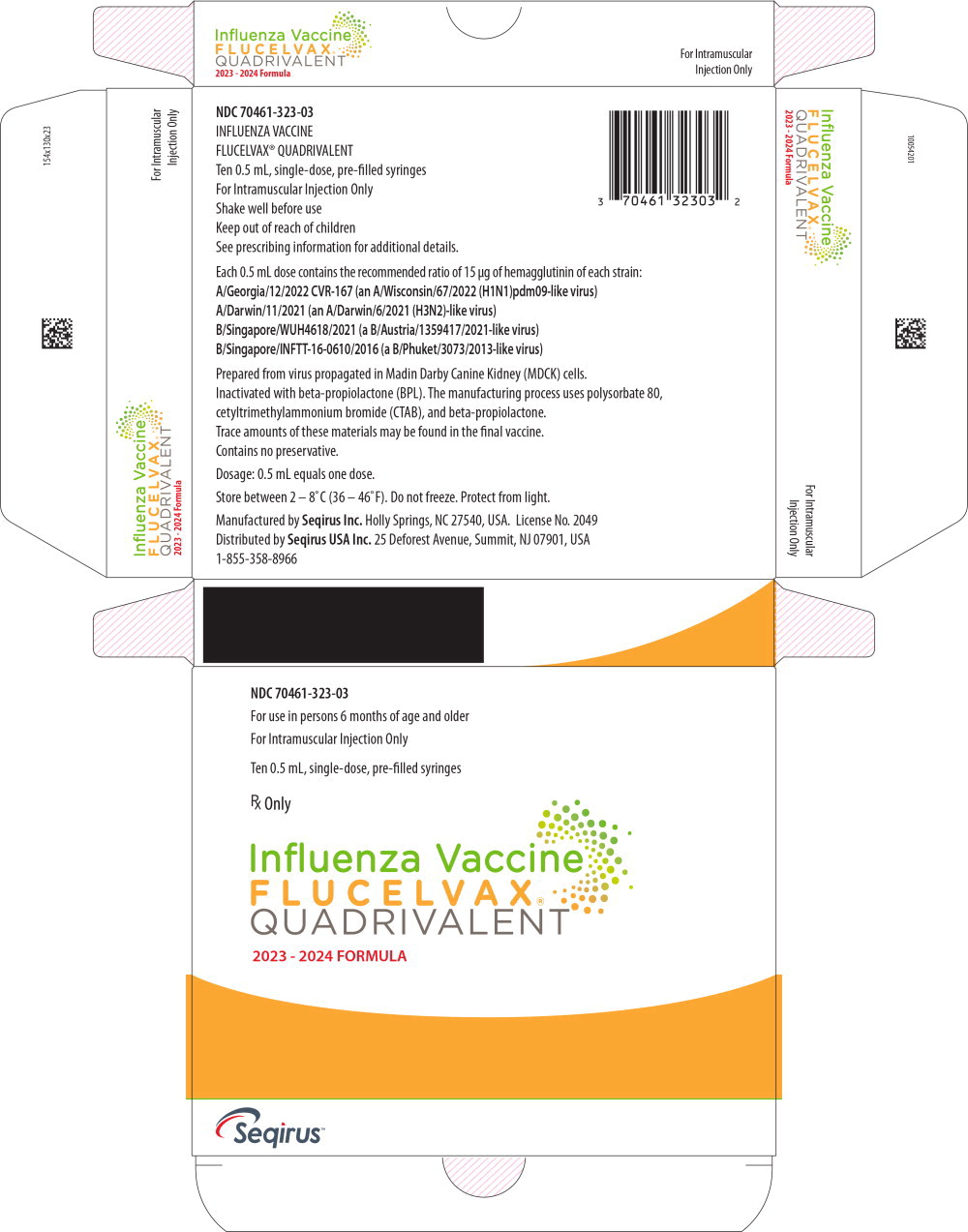
These highlights do not include all the information needed to use FLUCELVAX ®QUADRIVALENT safely and effectively. See full prescribing information for FLUCELVAX QUADRIVALENT. FLUCELVAX QUADRIVALENT (Influenza Vaccine) Suspension for Intramuscular Injection 2023-2024 Formula Initial U.S. Approval: 2016
Flucelvax Quadrivalent by
Drug Labeling and Warnings
Flucelvax Quadrivalent by is a Other medication manufactured, distributed, or labeled by Bamboo US BidCo LLC, Seqirus Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
FLUCELVAX QUADRIVALENT- influenza a virus a/georgia/12/2022 cvr-167 (h1n1) antigen (mdck cell derived, propiolactone inactivated), influenza a virus a/darwin/11/2021 (h3n2) antigen (mdck cell derived, propiolactone inactivated),influenza b virus b/singapore/wuh4618/2021 antigen (mdck cell derived, propiolactone inactivated),influenza b virus b/singapore/inftt-16-0610/2016 antigen (mdck cell derived, propiolactone inactivated) injection, suspension
Bamboo US BidCo LLC
----------
These highlights do not include all the information needed to use FLUCELVAX
®QUADRIVALENT safely and effectively. See full prescribing information for FLUCELVAX QUADRIVALENT.
FLUCELVAX QUADRIVALENT (Influenza Vaccine)
Suspension for Intramuscular Injection
2023-2024 Formula
Initial U.S. Approval: 2016
| FLUCELVAX QUADRIVALENT
influenza a virus a/georgia/12/2022 cvr-167 (h1n1) antigen (mdck cell derived, propiolactone inactivated), influenza a virus a/darwin/11/2021 (h3n2) antigen (mdck cell derived, propiolactone inactivated),influenza b virus b/singapore/wuh4618/2021 antigen (mdck cell derived, propiolactone inactivated),influenza b virus b/singapore/inftt-16-0610/2016 antigen (mdck cell derived, propiolactone inactivated) injection, suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Bamboo US BidCo LLC (119087615) |
Revised: 2/2026
Document Id: 49db6ede-b2eb-bddb-e063-6294a90afc64
Set id: 1a525ed7-5c81-f086-e063-6294a90a7797
Version: 5
Effective Time: 20260202
Trademark Results [Flucelvax Quadrivalent]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 FLUCELVAX QUADRIVALENT 79170996 4938921 Live/Registered |
Seqirus UK Limited 2015-07-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.