NUBEQA- darolutamide tablet, film coated
NUBEQA by
Drug Labeling and Warnings
NUBEQA by is a Prescription medication manufactured, distributed, or labeled by Bayer HealthCare Pharmaceuticals Inc., Orion Corporation, Orion Pharma, Sharp Corporation, Fermion Oy, Quinta-Analytica s.r.o.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NUBEQA safely and effectively. See full prescribing information for NUBEQA.
NUBEQA (darolutamide) tablets, for oral use
Initial U.S. Approval: 2019INDICATIONS AND USAGE
NUBEQA is an androgen receptor inhibitor indicated for the treatment of patients with non-metastatic castration-resistant prostate cancer. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Tablets: 300 mg (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most common adverse reactions (≥2%) are fatigue, pain in extremity, and rash. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Bayer HealthCare Pharmaceuticals Inc. at 1-888-842-2937 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Combined P-gp and Strong or Moderate CYP3A Inducers: Avoid concomitant use. (7.1)
- Combined P-gp and Strong CYP3A Inhibitors: Monitor patients more frequently for NUBEQA adverse reactions. (7.1)
- BCRP Substrates: Avoid concomitant use with drugs that are BCRP substrates where possible. If used together, monitor patients more frequently for adverse reactions and consider dose reduction of the BCRP substrate drug. (7.2).
USE IN SPECIFIC POPULATIONS
Severe Renal Impairment (not on hemodialysis): Recommended dose is 300 mg twice daily. (8.6)
Moderate Hepatic Impairment: Recommended dose is 300 mg twice daily. (8.7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 7/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dosage Modification
2.3 Recommended Dosage in Patients with Severe Renal Impairment
2.4 Recommended Dosage in Patients with Moderate Hepatic Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on NUBEQA
7.2 Effects of NUBEQA on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
The recommended dose of NUBEQA is 600 mg (two 300 mg film-coated tablets) taken orally, twice daily, equivalent to a total daily dose of 1200 mg. Swallow tablets whole with food [see Clinical Pharmacology (12.3)].
Patients receiving NUBEQA should also receive a gonadotropin-releasing hormone (GnRH) analog concurrently or should have had a bilateral orchiectomy.
Advise patients to take any missed dose as soon as they remember prior to the next scheduled dose, and not to take two doses together to make up for a missed dose.
2.2 Dosage Modification
If a patient experiences a greater than or equal to Grade 3 toxicity or an intolerable adverse reaction, withhold dosing or reduce to 300 mg twice daily until symptoms improve. Then the treatment may be resumed at a dose of 600 mg twice daily.
Dose reduction below 300 mg twice daily is not recommended.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
The safety and efficacy of NUBEQA have not been established in females. Based on its mechanism of action, NUBEQA can cause fetal harm and loss of pregnancy when administered to a pregnant female [see Clinical Pharmacology (12.1)].
Advise males with female partners of reproductive potential to use effective contraception during treatment and for 1 week after the last dose of NUBEQA [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
ARAMIS, a randomized (2:1), double-blind, placebo-controlled, multi-center clinical study, enrolled patients who had non-metastatic, castration-resistant prostate cancer (nmCRPC). In this study, patients received either NUBEQA at a dose of 600 mg, or a placebo, twice a day. All patients in the ARAMIS study received a concomitant gonadotropin-releasing hormone (GnRH) analog or had a bilateral orchiectomy. The median duration of exposure was 14.8 months (range: 0 to 44.3 months) in patients who received NUBEQA.
Overall, serious adverse reactions occurred in 25% of patients receiving NUBEQA and in 20% of patients receiving placebo. Serious adverse reactions in ≥ 1 % of patients who received NUBEQA included urinary retention, pneumonia and hematuria. Overall 3.9% of patients receiving NUBEQA and 3.2% of patients receiving placebo died from adverse reactions, which included death (0.4%), cardiac failure (0.3%), cardiac arrest (0.2%), general physical health deterioration (0.2%), and pulmonary embolism (0.2%) for NUBEQA.
Permanent discontinuation due to adverse reactions occurred in 9% of patients receiving NUBEQA or placebo. The most frequent adverse reactions requiring permanent discontinuation in patients who received NUBEQA included cardiac failure (0.4%), and death (0.4%).
Dosage interruptions due to adverse reactions occurred in 13% of patients treated with NUBEQA. The most frequent adverse reactions requiring dosage interruption in patients who received NUBEQA included hypertension (0.6%), diarrhea (0.5%), and pneumonia (0.5%).
Dosage reductions due to adverse reactions occurred in 6% of patients treated with NUBEQA. The most frequent adverse reactions requiring dosage reduction in patients treated with NUBEQA included fatigue (0.7%), hypertension (0.3%), and nausea (0.3%).
Table 1 shows adverse reactions in ARAMIS reported in the NUBEQA arm with a ≥2% absolute increase in frequency compared to placebo. Table 2 shows laboratory test abnormalities related to NUBEQA treatment and reported more frequently in NUBEQA-treated patients compared to placebo-treated patients in the ARAMIS study.
Table 1: Adverse Reactions in ARAMIS - * Common Terminology Criteria for Adverse Events (CTCAE) version 4.03
- † Includes fatigue and asthenia
Adverse Reaction*
NUBEQA
(n=954)
Placebo
(n=554)
All Grades
%Grades > 3
%
All Grades
%Grade > 3
%Fatigue†
16
0.6
11
1.1
Pain in extremity
6
0
3
0.2
Rash
3
0.1
1
0
Additionally, clinically significant adverse reactions occurring in 2% or more of patients treated with NUBEQA included ischemic heart disease (4.0% versus 3.4% on placebo) and heart failure (2.1% versus 0.9% on placebo).
Table 2: Laboratory Test Abnormalities in ARAMIS Laboratory Abnormality
NUBEQA
(N=954)*Placebo
(N=554)*All Grades†
%Grade 3-4†
%All Grades†
%Grade 3-4†
%- * The denominator used to calculate the rate varied based on the number of patients with a baseline value and at least one post-treatment value.
- † Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.
Neutrophil count decreased
20
4
9
0.6
AST increased
23
0.5
14
0.2
Bilirubin increased
16
0.1
7
0
-
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on NUBEQA
Combined P-gp and Strong or Moderate CYP3A4 Inducer
Concomitant use of NUBEQA with a combined P-gp and strong or moderate CYP3A4 inducer decreases darolutamide exposure which may decrease NUBEQA activity [see Clinical Pharmacology (12.3)]. Avoid concomitant use of NUBEQA with combined P-gp and strong or moderate CYP3A4 inducers.
Combined P-gp and Strong CYP3A4 Inhibitors
Concomitant use of NUBEQA with a combined P-gp and strong CYP3A4 inhibitor increases darolutamide exposure [see Clinical Pharmacology (12.3)] which may increase the risk of NUBEQA adverse reactions. Monitor patients more frequently for NUBEQA adverse reactions and modify NUBEQA dosage as needed [see Dosage and Administration (2.2)].
7.2 Effects of NUBEQA on Other Drugs
Breast Cancer Resistance Protein (BCRP) Substrates
NUBEQA is an inhibitor of BCRP transporter. Concomitant use of NUBEQA increases the AUC and Cmax of BCRP substrates [see Clinical Pharmacology (12.3)], which may increase the risk of BCRP substrate-related toxicities.
Avoid concomitant use with drugs that are BCRP substrates where possible. If used together, monitor patients more frequently for adverse reactions, and consider dose reduction of the BCRP substrate drug. Consult the approved product labeling of the BCRP substrate when used concomitantly with NUBEQA.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The safety and efficacy of NUBEQA have not been established in females. Based on its mechanism of action, NUBEQA can cause fetal harm and loss of pregnancy [see Clinical Pharmacology (12.1)]. Animal embryo-fetal developmental toxicology studies were not conducted with darolutamide. There are no human data on the use of NUBEQA in pregnant females.
8.3 Females and Males of Reproductive Potential
Contraception
Males
Based on the mechanism of action, advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 1 week after the last dose of NUBEQA [see Use in Specific Populations (8.1)].
Infertility
Males
Based on animal studies, NUBEQA may impair fertility in males of reproductive potential [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness of NUBEQA in pediatric patients have not been established.
8.5 Geriatric Use
Of the 954 patients who received NUBEQA in ARAMIS, 88% of patients were 65 years and over, and 49% were 75 years and over. No overall differences in safety or efficacy were observed between these patients and younger patients.
8.6 Renal Impairment
Patients with severe renal impairment (eGFR 15–29 mL/min/1.73 m2) who are not receiving hemodialysis have a higher exposure to NUBEQA and reduction of the dose is recommended [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)]. No dose reduction is needed for patients with mild or moderate renal impairment (eGFR 30-89 mL/min/1.73 m2). The effect of end stage renal disease (eGFR ≤15 mL/min/1.73 m2) on darolutamide pharmacokinetics is unknown.
8.7 Hepatic Impairment
Patients with moderate hepatic impairment (Child-Pugh Class B) have a higher exposure to NUBEQA and reduction of the dose is recommended [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)]. No dose reduction is needed for patients with mild hepatic impairment. The effect of severe hepatic impairment (Child-Pugh C) on darolutamide pharmacokinetics is unknown.
-
10 OVERDOSAGE
There is no known specific antidote for darolutamide overdose. The highest dose of NUBEQA studied clinically was 900 mg twice daily, equivalent to a total daily dose of 1800 mg. No dose limiting toxicities were observed with this dose.
Considering the saturable absorption and the absence of evidence for acute toxicity, an intake of a higher than recommended dose of darolutamide is not expected to lead to systemic toxicity in patients with intact hepatic and renal function [see Clinical Pharmacology (12.3)].
In the event of intake of a higher than recommended dose in patients with severe renal impairment or moderate hepatic impairment, if there is suspicion of toxicity, interrupt NUBEQA treatment and undertake general supportive measures until clinical toxicity has been diminished or resolved. If there is no suspicion of toxicity, NUBEQA treatment can be continued with the next dose as scheduled.
-
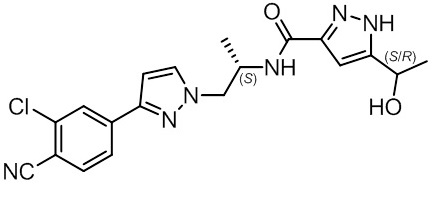
11 DESCRIPTION
NUBEQA is an androgen receptor inhibitor. The chemical name is N-{(2S)-1-[3-(3-chloro-4-cyanophenyl)-1H-pyrazol-1-yl]propan-2-yl}-5-(1-hydroxyethyl)-1H-pyrazole-3-carboxamide.
- The molecular weight is 398.85 and the molecular formula is C19H19Cl N6O2. The structural formula is:
Darolutamide is an optically active with a specific rotation value [α]20D= 72.2 o*mL/(dm*g), white to greyish- or yellowish white crystalline powder, that is soluble in tetrahydrofuran, but practically insoluble in aqueous medium. Darolutamide has a pKa of 11.75.
NUBEQA (darolutamide) is supplied as film-coated tablets containing 300 mg of darolutamide for oral use. The inactive ingredients of the tablet are: calcium hydrogen phosphate, croscarmellose sodium, lactose monohydrate, magnesium stearate, povidone K 30, hypromellose 15 cP, macrogol 3350, and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Darolutamide is an androgen receptor (AR) inhibitor. Darolutamide competitively inhibits androgen binding, AR nuclear translocation, and AR-mediated transcription. A major metabolite, keto-darolutamide, exhibited similar in vitro activity to darolutamide. In addition, darolutamide functioned as a progesterone receptor (PR) antagonist in vitro (approximately 1% activity compared to AR). Darolutamide decreased prostate cancer cell proliferation in vitro and tumor volume in mouse xenograft models of prostate cancer.
12.2 Pharmacodynamics
Darolutamide exposure at 600 mg twice daily results in PSA mean reduction of more than 90% from baseline.
12.3 Pharmacokinetics
Following administration of 600 mg twice daily, darolutamide mean (%CV) steady-state peak plasma concentration (Cmax) is 4.79 mg/L (30.9%) and area under the plasma concentration-time curve from time 0 to 12 hours (AUC12h) is 52.82 hµg/mL (33.9%). Steady-state is reached 2–5 days after repeated dosing with food, with an approximate 2-fold accumulation.
The exposure (Cmax and AUC12) of the darolutamide and the active metabolite keto‑darolutamide increase in a nearly dose-proportional manner in the dose range of 100 to 700 mg (0.17 to 1.17 times the approved recommended dosage). No further increase in darolutamide exposure was observed at 900 mg twice daily (1.5 times the approved recommended dosage).
Absorption
Darolutamide Cmax is reached approximately 4 hours after administration of a single 600 mg oral dose.
The absolute bioavailability is approximately 30% following oral administration of a NUBEQA tablet containing 300 mg darolutamide under fasted conditions.
Food Effect
Bioavailability of darolutamide increased by 2.0 to 2.5‑fold when administered with food. A similar increase of exposure was observed for the active metabolite keto‑darolutamide.
Distribution
The apparent volume of distribution of darolutamide after intravenous administration is 119 L.
Protein binding is 92% for darolutamide and 99.8% for the active metabolite, keto‑darolutamide. Serum albumin is the main binding protein for darolutamide and keto-darolutamide.
Elimination
The effective half-life of darolutamide and keto‑darolutamide is approximately 20 hours in patients. The clearance (%CV) of darolutamide following intravenous administration is 116 mL/min (39.7%).
Metabolism
Darolutamide is primarily metabolized by CYP3A4, as well as by UGT1A9 and UGT1A1. Keto‑darolutamide total exposure in plasma is 1.7‑fold higher compared to darolutamide.
Excretion
After a single radiolabeled dose as an oral solution, a total of 63.4% of darolutamide‑related material is excreted in the urine (approximately 7% unchanged) and 32.4% (approximately 30% unchanged) in the feces. More than 95% of the dose was recovered within 7 days after administration.
Specific Populations
In nmCRPC patients, no clinically significant differences in the pharmacokinetics of darolutamide were observed based on age (48-95 years), race (White, Japanese, non-Japanese Asian, Black or African American), mild to moderate renal impairment (eGFR 30–89 mL/min/1.73m2), or mild hepatic impairment.
In non-cancer subjects with severe renal impairment (eGFR 15–29 mL/min/1.73 m2) not receiving dialysis or with moderate hepatic impairment (Child-Pugh Class B), NUBEQA exposure increased by about 2.5- and 1.9-fold, respectively, compared to healthy subjects.
The effect of end-stage renal disease (eGFR <15 mL/min/1.73 m2) or severe hepatic impairment (Child-Pugh C) on darolutamide pharmacokinetics has not been studied.
Drug Interaction Studies
Clinical Studies
Combined P-gp and Strong CYP3A4 Inducers
Concomitant use of rifampicin (a combined P-gp and strong CYP3A4 inducer) decreased mean darolutamide AUC0-72 by 72% and Cmax by 52%. The decrease of darolutamide exposure by moderate CYP3A4 inducers is expected to be in the range of 36% – 58 %.
Combined P-gp and Strong CYP3A4 Inhibitors
Itraconazole (a strong combined CYP3A4 and P-gp inhibitor) increased mean darolutamide AUC0-72 by 1.7- and Cmax by 1.4-fold.
CYP3A4 substrates
Concomitant use of darolutamide decreased the mean AUC and Cmax of midazolam (CYP3A4 substrate) by 29% and 32%, respectively. No clinically significant differences in the pharmacokinetics of midazolam were observed when used concomitantly with darolutamide.
BCRP Substrates
Concomitant use of darolutamide increased the mean AUC and Cmax of rosuvastatin (BCRP substrate) by approximately 5-fold.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies to evaluate the carcinogenic potential of darolutamide have not been conducted.
Darolutamide was clastogenic in an in vitro chromosome aberration assay in human peripheral blood lymphocytes. Darolutamide did not induce mutations in the bacterial reverse mutation (Ames) assay and was not genotoxic in the in vivo combined bone marrow micronucleus assay and the Comet assay in the liver and duodenum of the rat.
Fertility studies in animals have not been conducted with darolutamide. In repeat-dose toxicity studies in male rats (up to 26 weeks) and dogs (up to 39 weeks), tubular dilatation of testes, hypospermia, and atrophy of seminal vesicles, testes, prostate gland and epididymides were observed at doses ≥ 100 mg/kg/day in rats (0.6 times the human exposure based on AUC) and ≥ 50 mg/kg/day in dogs (approximately 1 times the human exposure based on AUC).
-
14 CLINICAL STUDIES
ARAMIS (NCT02200614) was a multicenter, double-blind, placebo-controlled clinical trial in 1509 patients with non-metastatic castration resistant prostate cancer with a prostate-specific antigen doubling time (PSADT) of ≤ 10 months. Randomization was stratified by PSADT and use of bone-targeted therapy at study entry. Patients with pelvic lymph nodes less than 2 cm in short axis below the aortic bifurcation were allowed to enter the study. Patients with a history of seizures were not excluded. Absence or presence of metastasis was assessed by blinded independent central review (BICR). PSA results were not blinded and were not used for treatment discontinuation.
Patients were randomized 2:1 to receive either 600 mg darolutamide orally twice daily (n=955) or matching placebo (n=554). Treatment continued until radiographic disease progression as assessed by CT, MRI, 99mTc bone scan by BICR, unacceptable toxicity or withdrawal. All patients received a gonadotropin-releasing hormone (GnRH) analog concurrently or had a bilateral orchiectomy.
The following patient demographics and disease characteristics were balanced between treatment arms. The median age was 74 years (range 48–95) and 9% of patients were 85 years of age or older. The racial distribution was 79% White, 13% Asian, and 3% Black. A majority of patients (73%) had a Gleason score of 7 or higher at diagnosis. The median PSADT was 4.5 months. Forty-two percent of patients in both treatment arms had prior surgery or radiotherapy to the prostate. Eleven percent of patients had enlarged pelvic lymph nodes less than 2 cm at study entry. Six percent of patients were retrospectively identified by BICR as having metastases at baseline. Seventy-three percent of patients received prior treatment with an anti-androgen (bicalutamide or flutamide). All patients had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) score of 0 or 1 at study entry. There were 12 patients enrolled on the NUBEQA arm with a history of seizure. At baseline, 47% of patients reported no pain on the Brief Pain Inventory-Short Form (a 7-day diary average of the daily worst pain item).
The major efficacy endpoint was metastasis free survival (MFS), defined as the time from randomization to the time of first evidence of BICR-confirmed distant metastasis or death from any cause within 33 weeks after the last evaluable scan, whichever occurred first. Distant metastasis was defined as new bone or soft tissue lesions or enlarged lymph nodes above the aortic bifurcation. Overall survival (OS) and time to pain progression were additional efficacy endpoints.
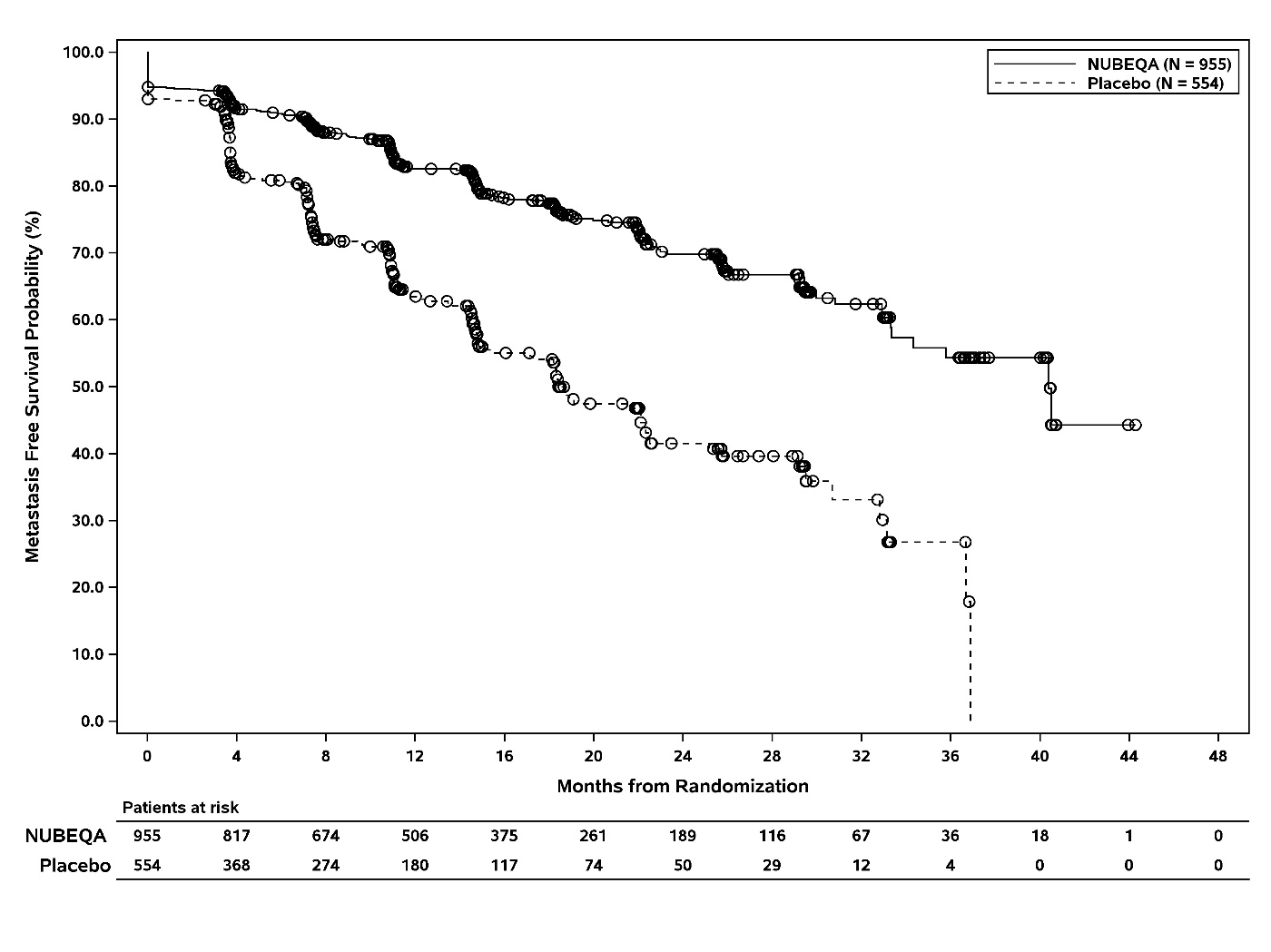
The efficacy results for MFS from ARAMIS are summarized in Table 3 and Figure 1. Treatment with NUBEQA resulted in a statistically significant improvement in MFS compared to placebo. MFS results were consistent across patient subgroups for PSADT (≤ 6 months or > 6 months) or prior use of bone-targeting agents (yes or no). OS data were not mature at the time of final MFS analysis (57% of the required number of events). Locoregional-only progression occurred in 6% of patients overall.
Table 3: Efficacy Results from the ARAMIS Study - * Based on Kaplan-Meier estimates
- † NR: not reached
- ‡ Hazard ratio is based on a Cox regression model (with treatment as the only covariate) stratified by PSADT (≤ 6 months vs. > 6 months) and use of osteoclast-targeted therapy (yes vs. no). Hazard ratio < 1 favors NUBEQA
- § P-value is based on a stratified log-rank test by PSADT (≤ 6 months vs. > 6 months) and use of osteoclast-targeted therapy (yes vs. no)
NUBEQA
(N=955)
Placebo
(N=554)
Metastasis-free survival
Number of Events (%)
221 (23)
216 (39)
Median, months (95% CI)*
40.4 (34.3, NR†)
18.4 (15.5, 22.3)
Hazard Ratio (95% CI)‡
0.41 (0.34, 0.50)
P-value§
<0.0001
The MFS result was supported by a delay in time to pain progression, defined as at least a 2-point worsening from baseline of the pain score on Brief Pain Inventory-Short Form or initiation of opioids, in patients treated with NUBEQA as compared to placebo. Pain progression was reported in 28% of all patients on study.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
NUBEQA (darolutamide) 300 mg film-coated tablets are white to off-white, oval shaped tablets, marked with “300” on one side, and “BAYER” on the other side. NUBEQA 300 mg tablets are available in bottles of 120 tablets.
NDC: 50419-395-01
-
17 PATIENT COUNSELING INFORMATION
Dosage and Administration
Inform patients receiving concomitant gonadotropin-releasing hormone (GnRH) analog therapy that they need to maintain this treatment during the course of treatment with NUBEQA.
Instruct patients to take their dose of two tablets (twice daily). NUBEQA should be taken with food. Each tablet should be swallowed whole.
Inform patients that in the event of a missed daily dose of NUBEQA, to take any missed dose, as soon as they remember prior to the next scheduled dose, and not to take two doses together to make up for a missed dose [see Dosage and Administration (2.1)].
Embryo-Fetal Toxicity
Inform patients that NUBEQA can be harmful to a developing fetus and can cause loss of pregnancy [see Use in Specific Populations (8.1)].
Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 1 week after the last dose of NUBEQA [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1, 8.3)].
Infertility
Advise male patients that NUBEQA may impair fertility [see Use in Specific Populations (8.3)].
Manufactured by: Orion Corporation, Orion Pharma, FI-02101 Espoo, Finland
Manufactured for: Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ 07981 USA
© 2019 Bayer HealthCare Pharmaceuticals Inc.
For more information, call Bayer HealthCare Pharmaceuticals Inc. at Bayer at 1-888-842-2937 or go to
www.NUBEQA-us.com -
Patient Package Insert
PATIENT INFORMATION
NUBEQA™ (NO͞O-bə-kə)
(darolutamide)
TabletsWhat is NUBEQA?
NUBEQA is a prescription medicine used to treat men with prostate cancer that has not spread to other parts of the body and no longer responds to a medical or surgical treatment that lowers testosterone.It is not known if NUBEQA is safe and effective in females.
It is not known if NUBEQA is safe and effective in children.
Before taking NUBEQA, tell your healthcare provider about all your medical conditions, including if you:
- have kidney or liver problems
- are pregnant or plan to become pregnant. NUBEQA can cause harm to your unborn baby and loss of pregnancy (miscarriage).
- have a partner who may become pregnant. Males who have female partners who may become pregnant should use effective birth control (contraception) during treatment and for 1 week after the last dose of NUBEQA. Talk with your healthcare provider about birth control methods.
- are breastfeeding or plan to breastfeed. It is not known if NUBEQA passes into breast milk.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. NUBEQA may affect the way other medicines work and other medicines may affect how NUBEQA works.
You should not start or stop any medicine before you talk with the healthcare provider that prescribed NUBEQA.
Know the medicines you take. Keep a list of them with you to show to your healthcare provider and pharmacist when you get a new medicine.
How should I take NUBEQA?
- Take NUBEQA exactly as your healthcare provider tells you.
- Your healthcare provider may change your dose if needed.
- Take your prescribed dose of NUBEQA 2 times a day with food.
- Swallow NUBEQA tablets whole.
- If you are receiving gonadotropin-releasing hormone (GnRH) analog therapy, you should continue with this treatment during your treatment with NUBEQA unless you have had a surgery to lower the amount of testosterone in your body (surgical castration).
- If you miss a dose of NUBEQA, take your prescribed dose as soon as you remember before the next scheduled dose. Do not take 2 doses together to make up for a missed dose.
What are the possible side effects of NUBEQA?
The most common side effects of NUBEQA include:
- feeling more tired than usual
- arm, leg, hand or foot pain
- rash
- decreased white blood cells (neutropenia)
- changes in liver function tests
NUBEQA may cause fertility problems in males, which may affect the ability to father children. Talk to your healthcare provider if you have concerns about fertility.
These are not all the possible side effects of NUBEQA.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store NUBEQA?
- Store NUBEQA at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep the bottle tightly closed after you first open it.
Keep NUBEQA and all medicines out of the reach of children.
General information about the safe and effective use of NUBEQA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use NUBEQA for a condition for which it was not prescribed. Do not give NUBEQA to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about NUBEQA that is written for health professionals.
What are the ingredients in NUBEQA?
Active ingredient:
darolutamide
Inactive ingredients:
calcium hydrogen phosphate, croscarmellose sodium, lactose monohydrate, magnesium stearate, povidone K 30, hypromellose 15 cP, macrogol 3350, and titanium dioxide.Manufactured by: Orion Corporation, Orion Pharma, FI-02101 Espoo, Finland
Manufactured for: Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ 07981 USA
© 2019 Bayer HealthCare Pharmaceuticals Inc.
For more information, call Bayer HealthCare Pharmaceuticals Inc. at Bayer at 1-888-842-2937 or go to www.NUBEQA-us.com
- This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: July 2019
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NUBEQA
darolutamide tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50419-395 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DAROLUTAMIDE (UNII: X05U0N2RCO) (DAROLUTAMIDE - UNII:X05U0N2RCO) DAROLUTAMIDE 300 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS DIBASIC CALCIUM PHOSPHATE (UNII: L11K75P92J) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE K30 (UNII: U725QWY32X) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (to off white) Score no score Shape OVAL Size 16mm Flavor Imprint Code 300;Bayer Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50419-395-01 1 in 1 CARTON 07/31/2019 1 120 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212099 07/31/2019 Labeler - Bayer HealthCare Pharmaceuticals Inc. (005436809) Establishment Name Address ID/FEI Business Operations Orion Corporation, Orion Pharma 539763727 MANUFACTURE(50419-395) Establishment Name Address ID/FEI Business Operations Sharp Corporation 143696495 PACK(50419-395) , LABEL(50419-395)
Trademark Results [NUBEQA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NUBEQA 87812407 5584749 Live/Registered |
Bayer Aktiengesellschaft 2018-02-27 |
 NUBEQA 77736052 3852172 Dead/Cancelled |
Bayer Aktiengesellschaft 2009-05-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.