These highlights do not include all the information needed to use HEPLISAV-B ®safely and effectively. See full prescribing information for HEPLISAV-B. HEPLISAV-B [Hepatitis B Vaccine (Recombinant), Adjuvanted] Solution for Intramuscular Injection Initial U.S. Approval: 2017
HEPLISAV-B by
Drug Labeling and Warnings
HEPLISAV-B by is a Other medication manufactured, distributed, or labeled by Bamboo US BidCo LLC, Dynavax Technologies Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
HEPLISAV-B- hepatitis b vaccine (recombinant) adjuvanted injection, solution
Bamboo US BidCo LLC
----------
These highlights do not include all the information needed to use HEPLISAV-B
®safely and effectively. See full prescribing information for HEPLISAV-B.
HEPLISAV-B [Hepatitis B Vaccine (Recombinant), Adjuvanted] Solution for Intramuscular Injection
Initial U.S. Approval: 2017
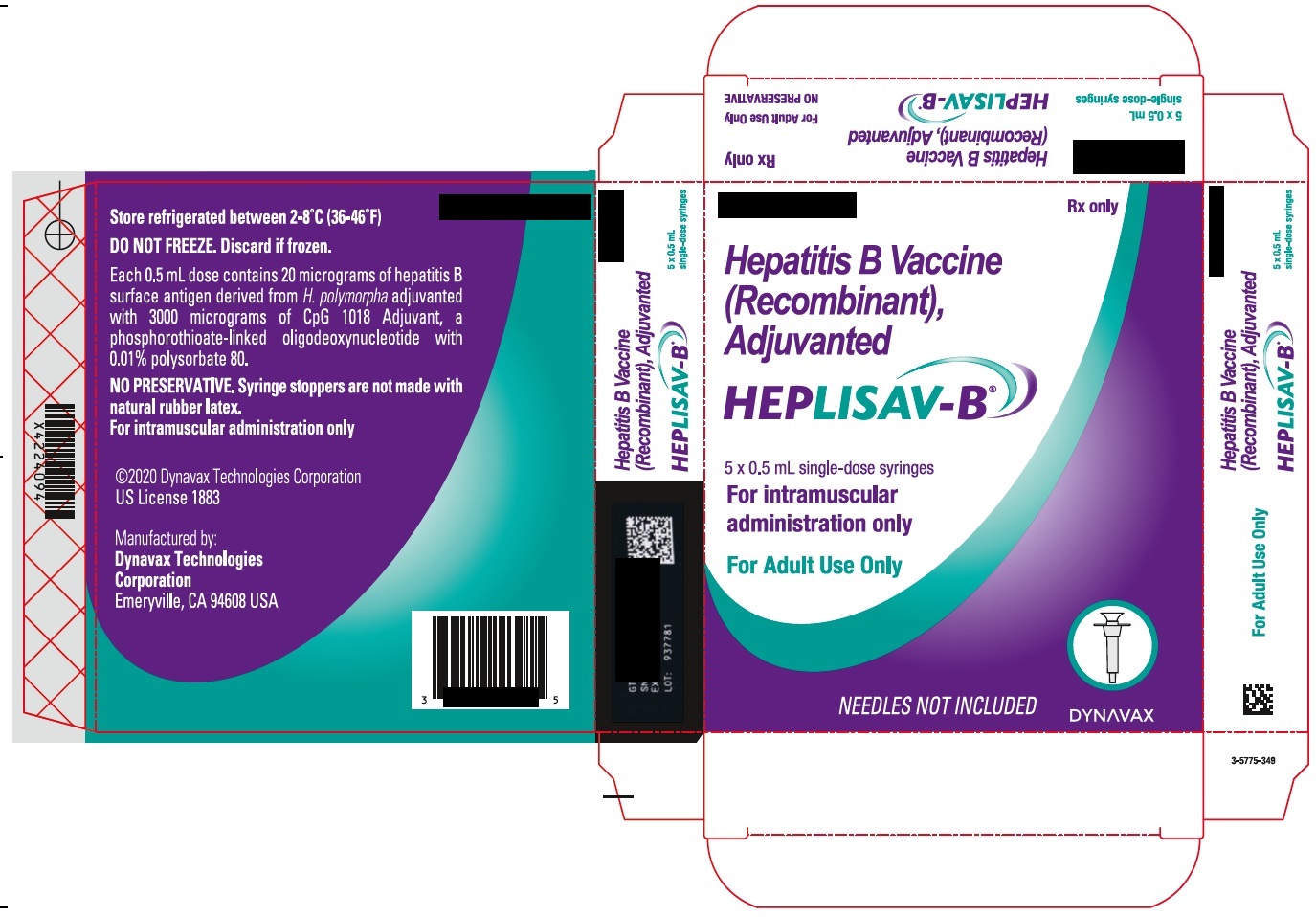
Principal Panel - Carton - Vials
Rx only
Hepatitis B Vaccine
(Recombinant),
Adjuvanted
HEPLISAV-B ™
5 x 0.5 mL
single-dose vials
For intramuscular
administration only
DYNAVAX
 |
| HEPLISAV-B
hepatitis b vaccine (recombinant) adjuvanted injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Bamboo US BidCo LLC (119087615) |
| Registrant - Dynavax Technologies Corporation (964173801) |
Revised: 6/2025
Document Id: 3750f1f3-e7d9-0d1d-e063-6394a90acd80
Set id: 1a8b8271-208d-ecde-e063-6294a90a54c6
Version: 3
Effective Time: 20250611
Trademark Results [HEPLISAV-B]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HEPLISAV-B 98014169 not registered Live/Pending |
Dynavax Technologies Corporation 2023-05-25 |
 HEPLISAV-B 98014166 not registered Live/Pending |
Dynavax Technologies Corporation 2023-05-25 |
 HEPLISAV-B 98014161 not registered Live/Pending |
Dynavax Technologies Corporation 2023-05-25 |
 HEPLISAV-B 87145972 5419134 Live/Registered |
Dynavax Technologies Corporation 2016-08-22 |
 HEPLISAV-B 85840674 not registered Dead/Abandoned |
Dynavax Technologies Corporation 2013-02-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.