SWFI Diluent for M-M-R ®II (Measles, Mumps, and Rubella Virus Vaccine Live) M-M-R ®II (Measles, Mumps, and Rubella Virus Vaccine Live) Suspension for intramuscular or subcutaneous injection Initial U.S. Approval: 1978 These highlights do not include all the information needed to use M-M-R II safely and effectively. See full prescribing information for M-M-R II.
M-M-R II by
Drug Labeling and Warnings
M-M-R II by is a Other medication manufactured, distributed, or labeled by Bamboo US BidCo LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
M-M-R II- measles, mumps, and rubella virus vaccine live injection, powder, lyophilized, for suspension
Bamboo US BidCo LLC
----------
SWFI Diluent for M-M-R ®II (Measles, Mumps, and Rubella Virus Vaccine Live)
M-M-R
®II (Measles, Mumps, and Rubella Virus Vaccine Live) Suspension for intramuscular or subcutaneous injection
Initial U.S. Approval: 1978
These highlights do not include all the information needed to use M-M-R II safely and effectively. See full prescribing information for M-M-R II.
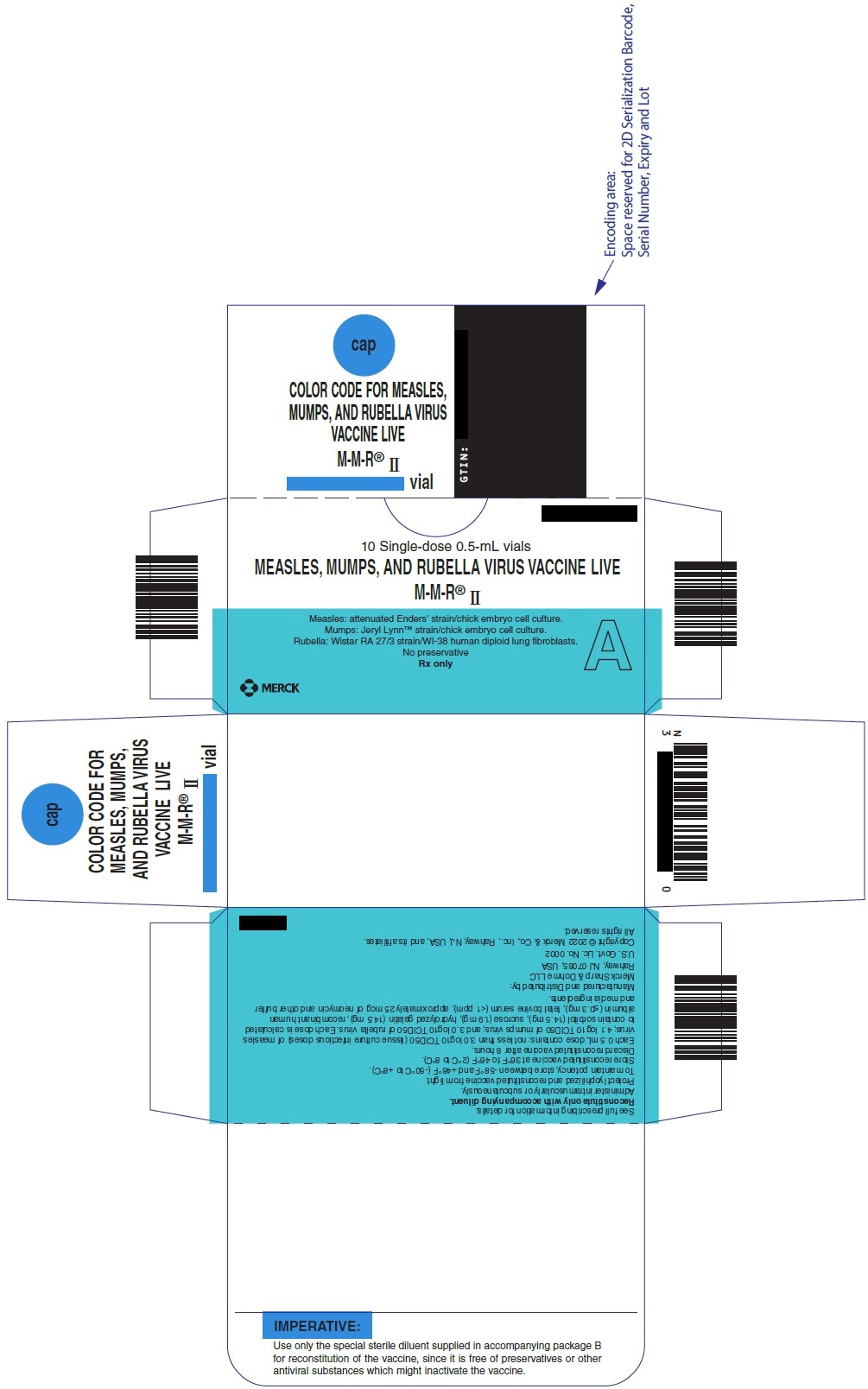
PRINCIPAL DISPLAY PANEL - 0.5 mL Vial Carton
NDC
10 Single-dose 0.5-mL vials
MEASLES, MUMPS, AND RUBELLA VIRUS VACCINE LIVE
M-M-R
®II
Measles: attenuated Enders' strain/chick embryo cell culture.
Mumps: Jeryl Lynn
™strain/chick embryo cell culture.
Rubella: Wistar RA 27/3 strain/WI-38 human diploid lung fibroblasts.
No preservative
Rx only
A
MERCK
| M-M-R II
measles, mumps, and rubella virus vaccine live injection, powder, lyophilized, for suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Bamboo US BidCo LLC (119087615) |
Revised: 12/2024
Document Id: 28eed024-f3c1-b00e-e063-6294a90aafc5
Set id: 1aa4f839-8b6e-4fc1-e063-6294a90afb75
Version: 4
Effective Time: 20241210
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.