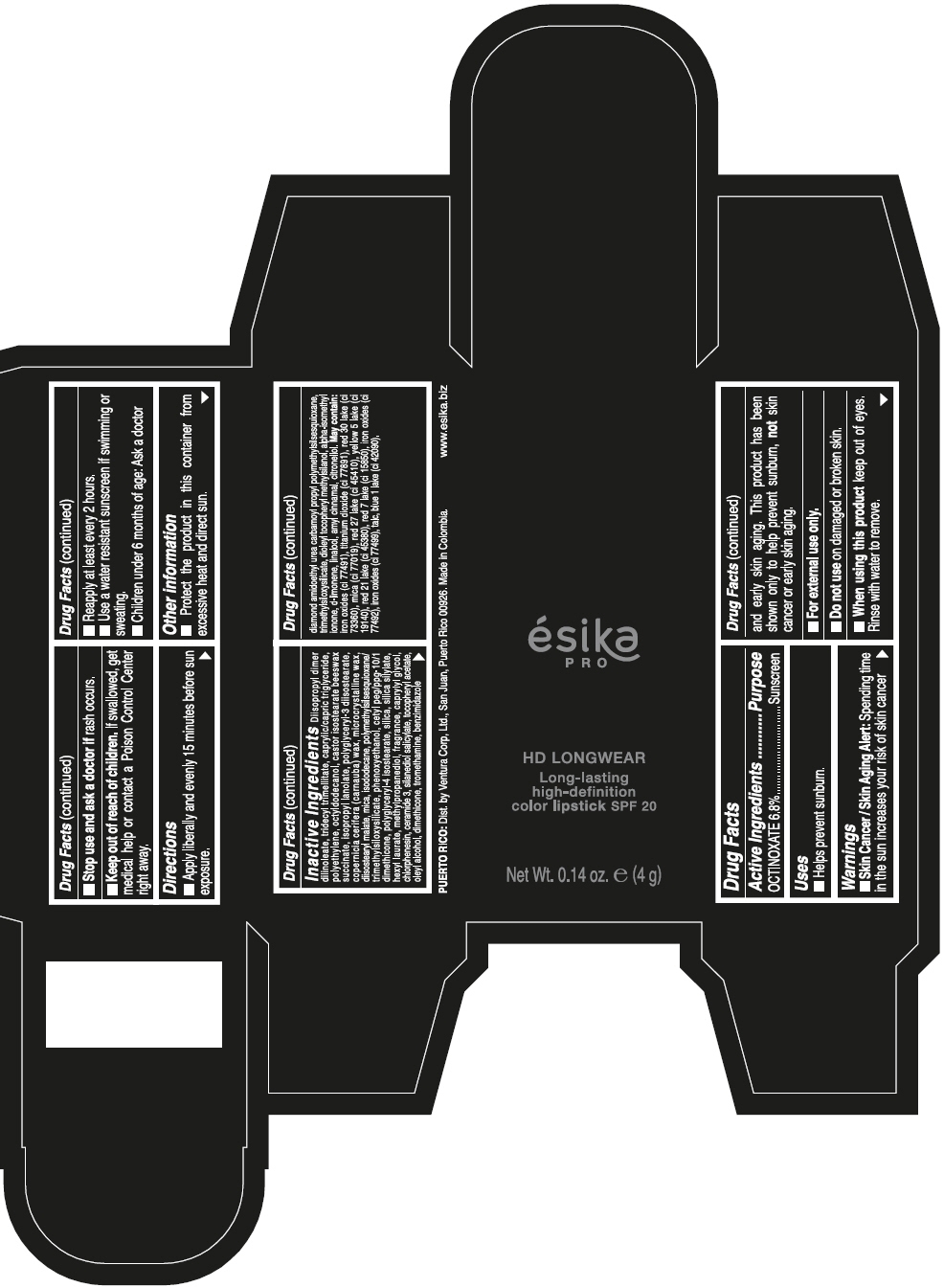
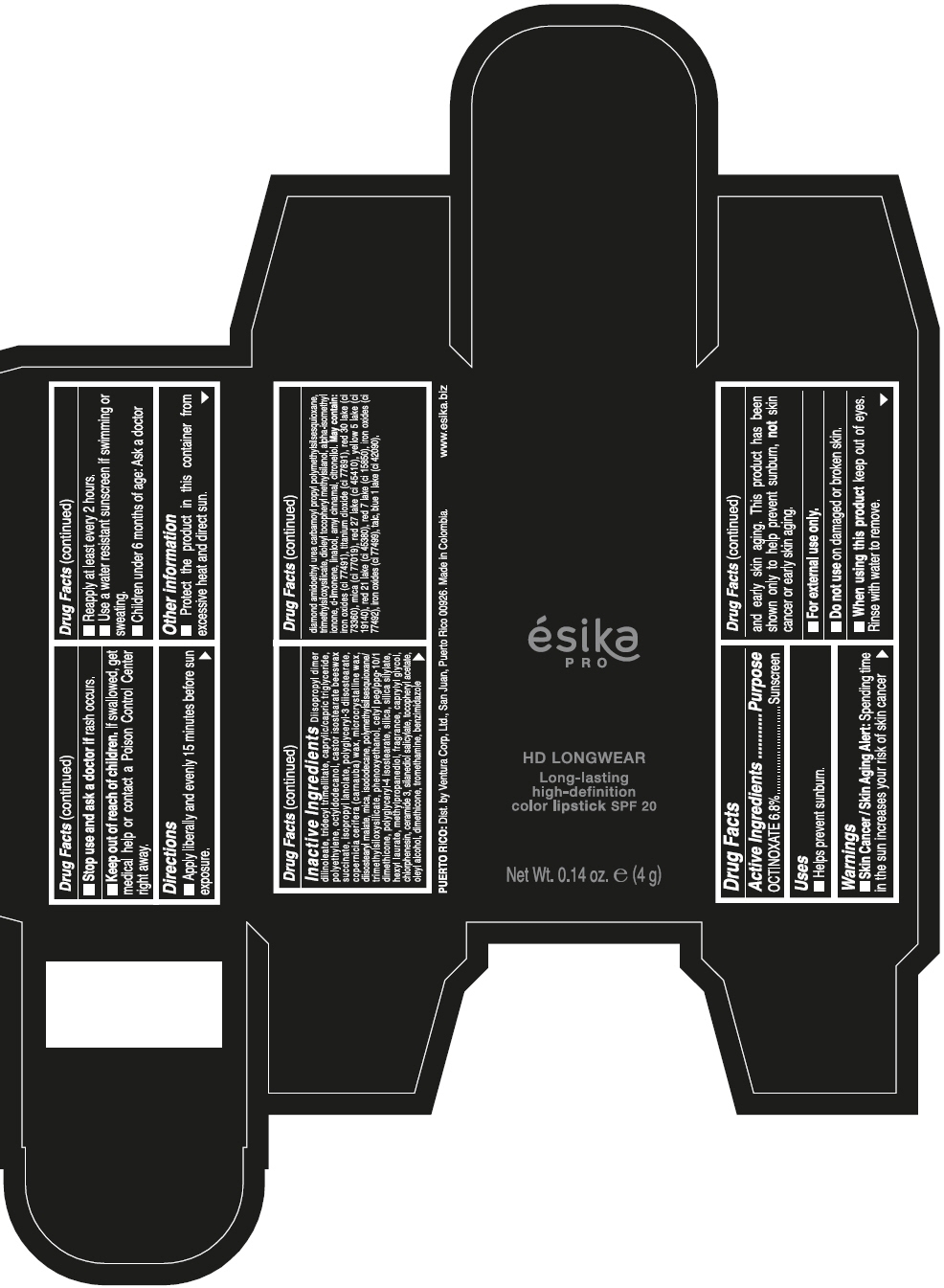
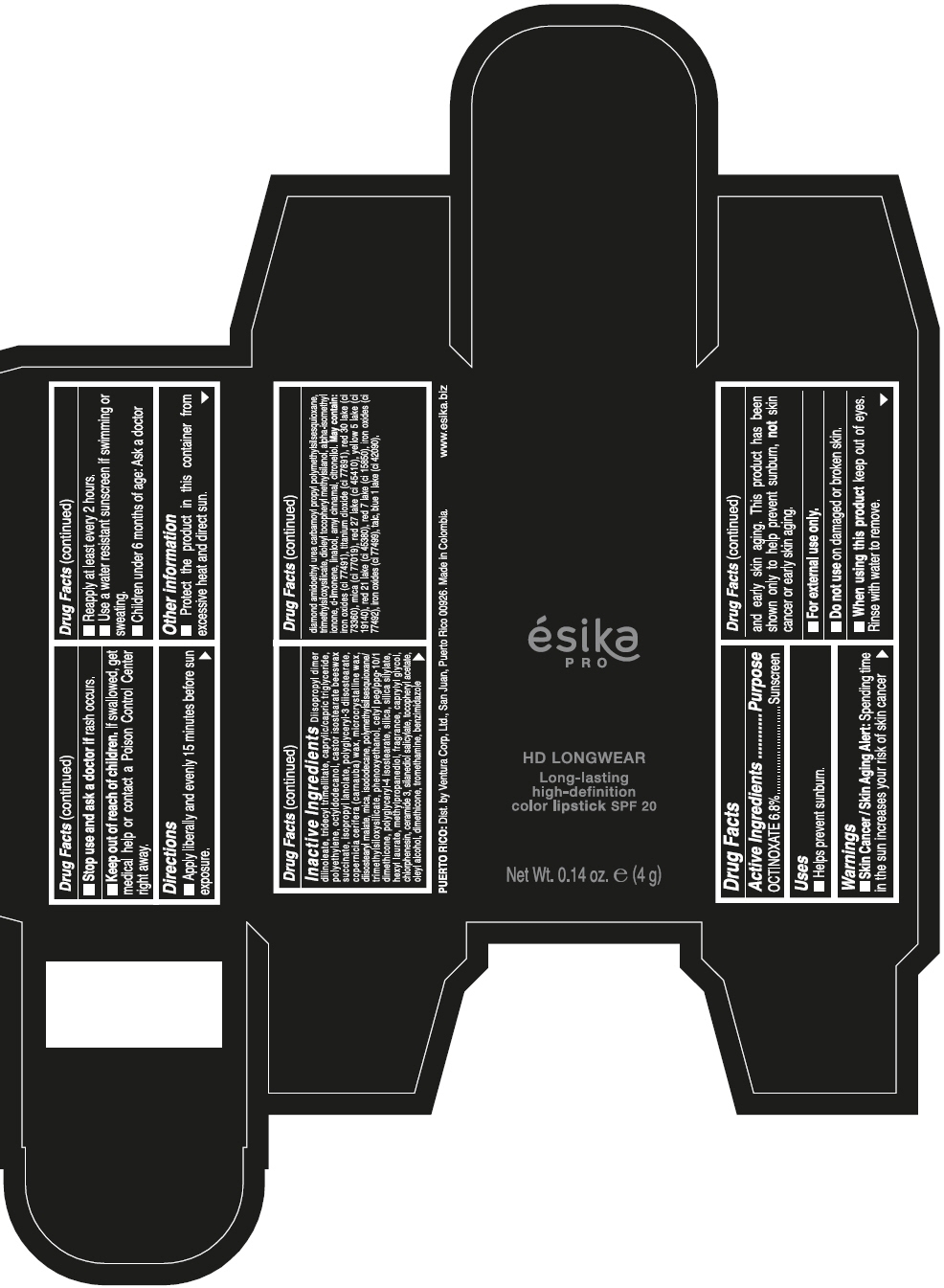
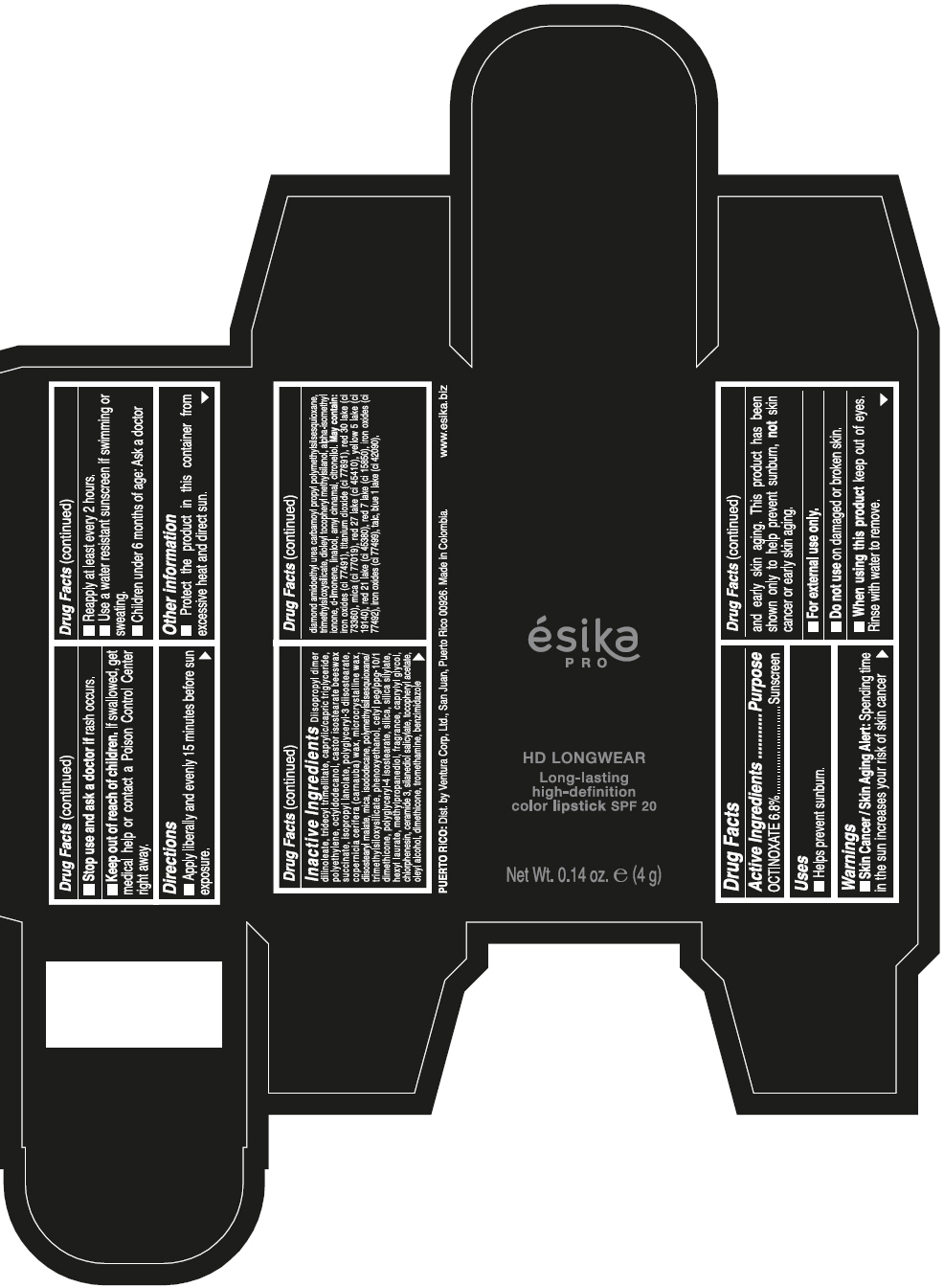
ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 VINO CAUTIVANTE - PURPLE- octinoxate lipstick ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 PIMIENTA CALIENTE - RED- octinoxate lipstick ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 ROJO GLAM - RED- octinoxate lipstick ESIKA PRO HD LONGWEAR LONG-LA .......TION COLOR SPF 20 MARRON HAVANA - BROWN- octinoxate lipstick ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 MARRON FANATIC - BROWN- octinoxate lipstick ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 NUDE ROSE- BEIGE- octinoxate lipstick ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20- octinoxate kit
Esika PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 by
Drug Labeling and Warnings
Esika PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 by is a Otc medication manufactured, distributed, or labeled by Ventura Corporation LTD, Bel Star S.A. (Colombia). Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
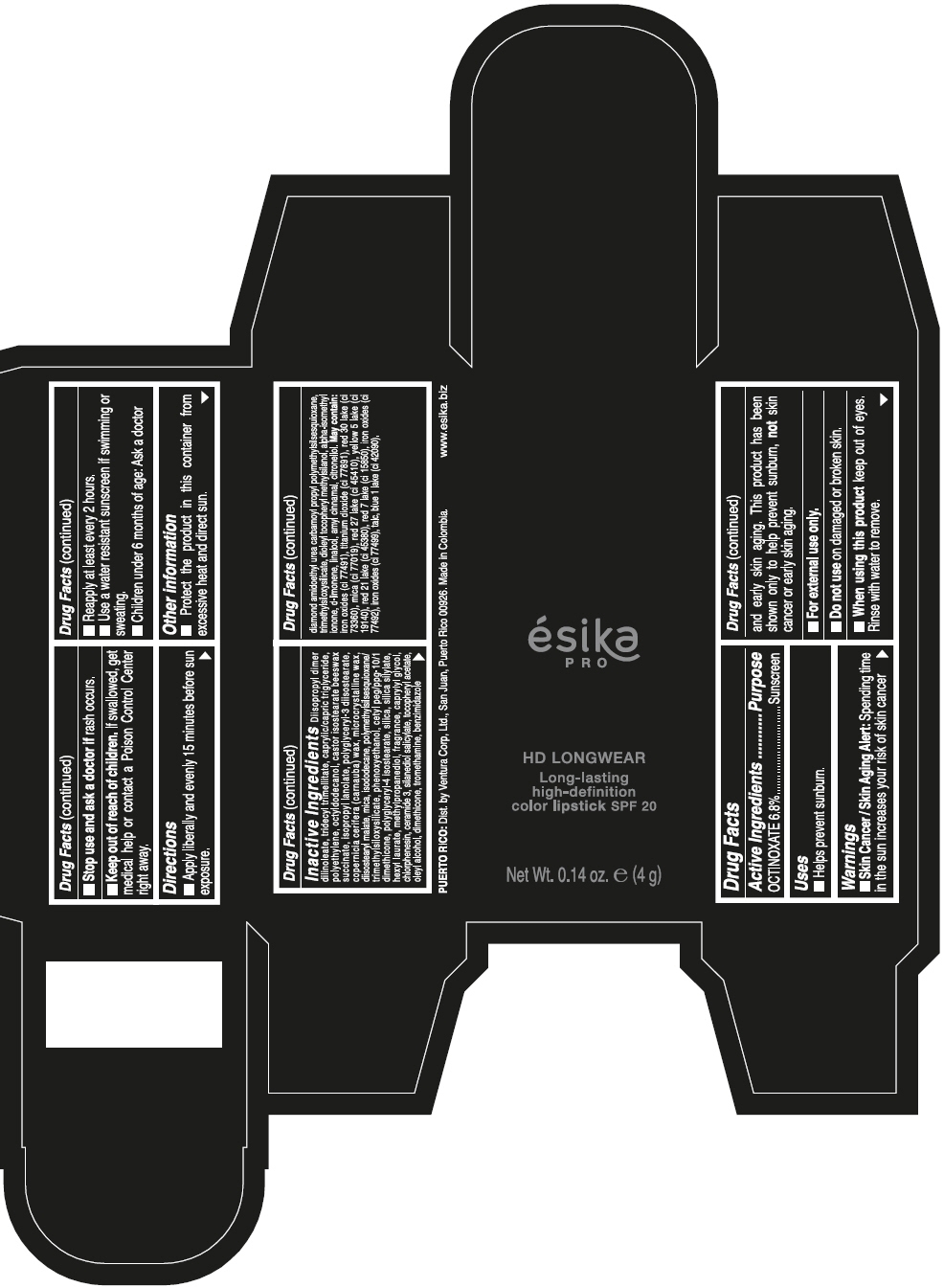
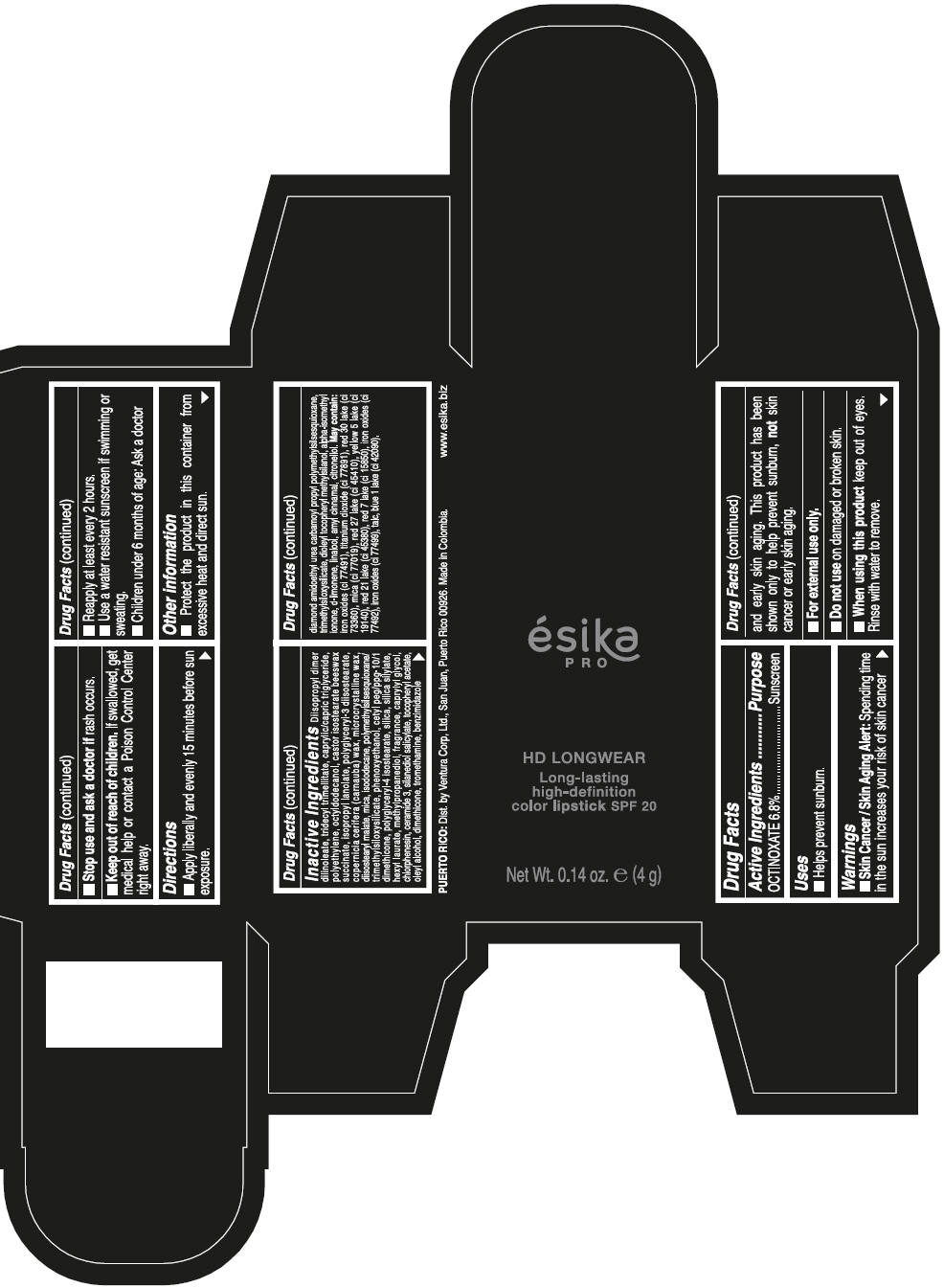
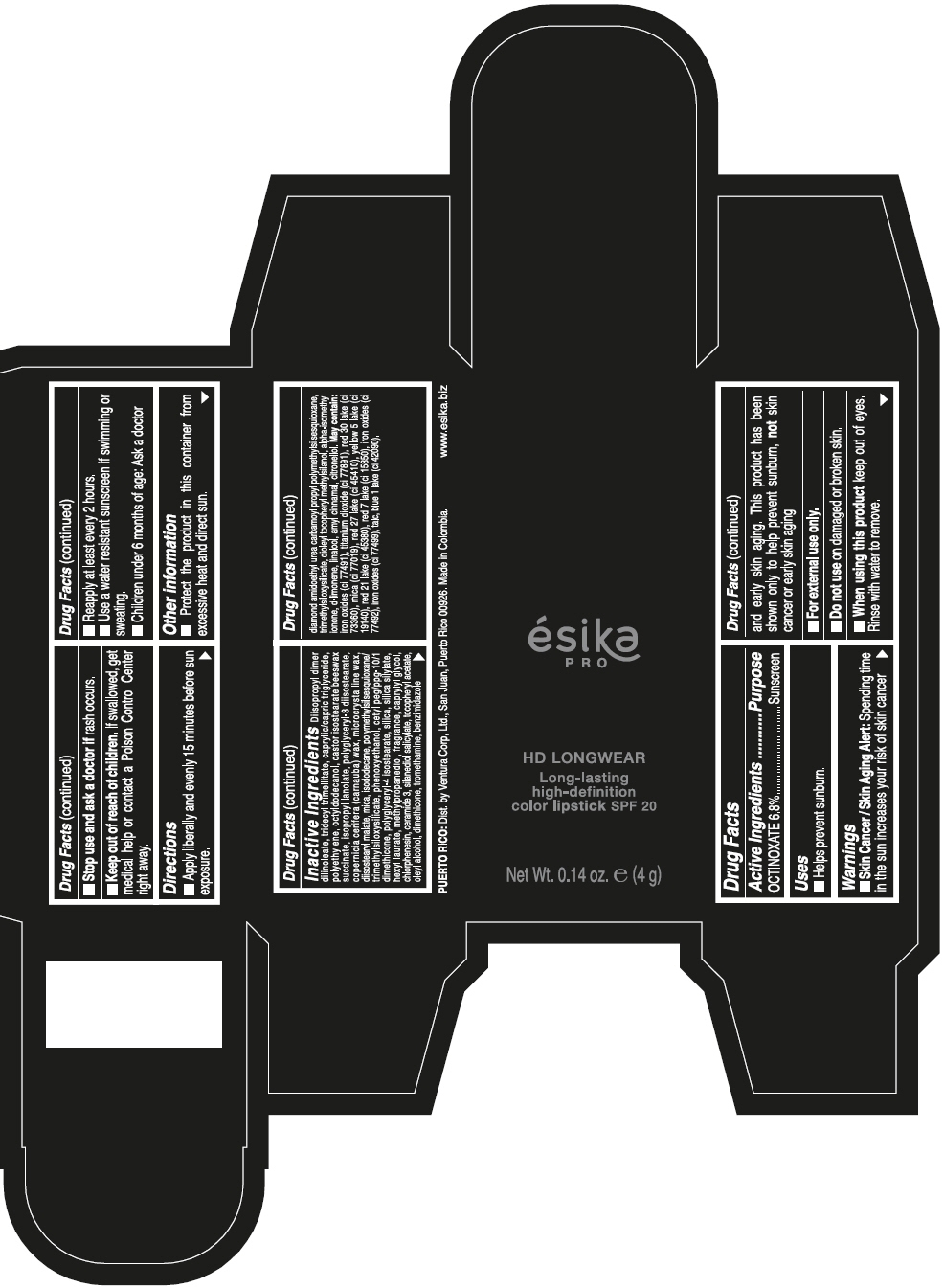
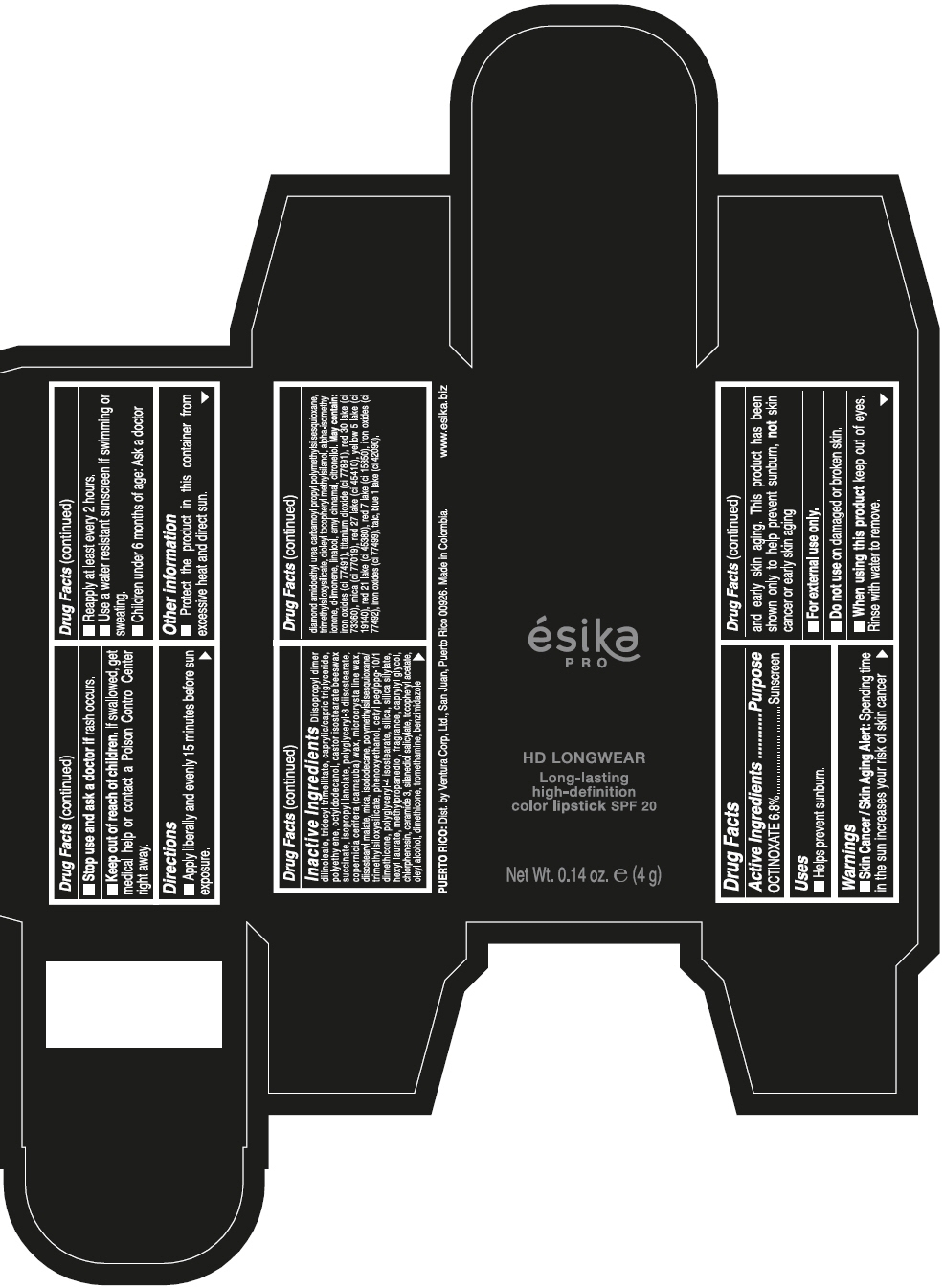
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
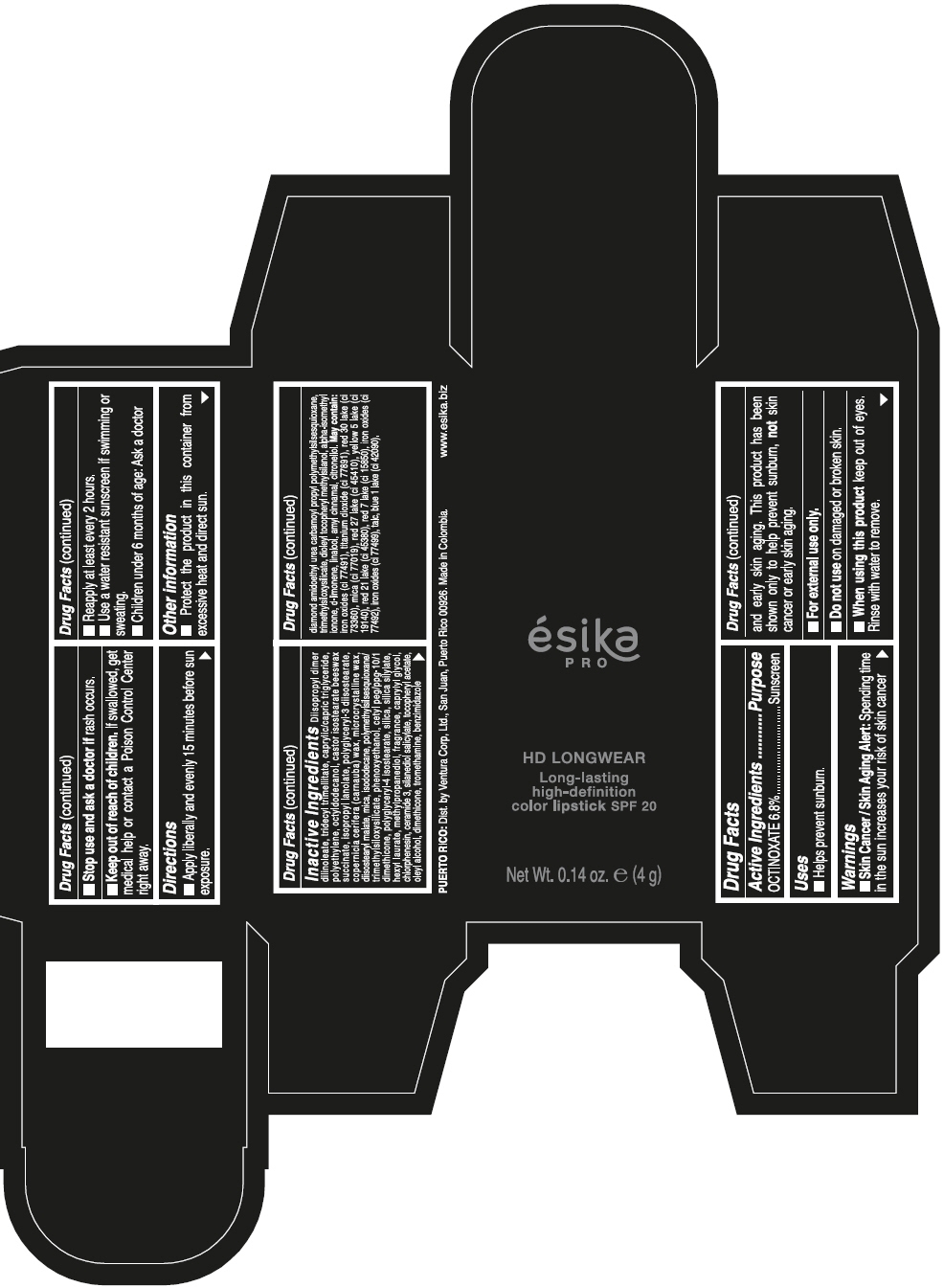
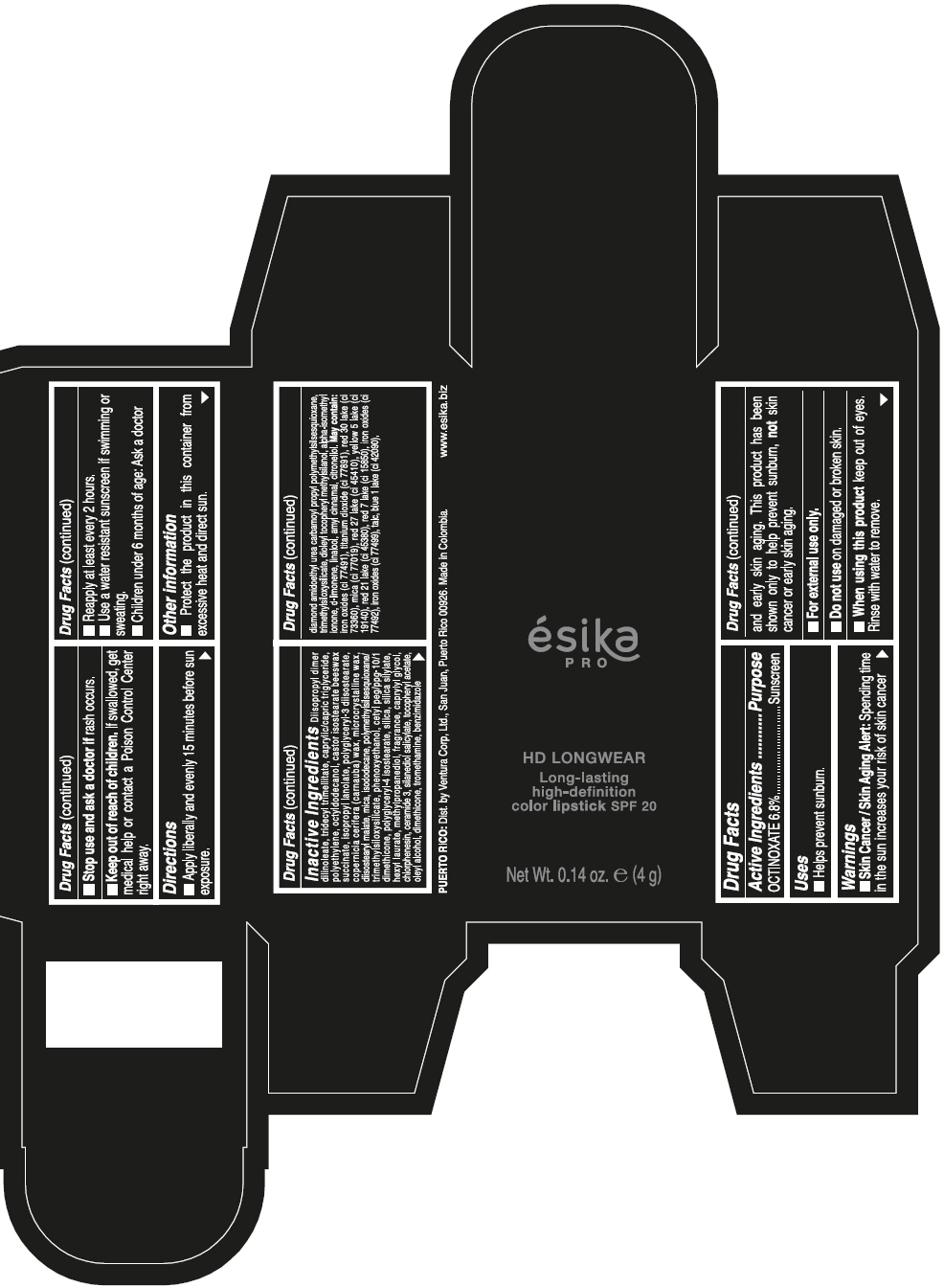
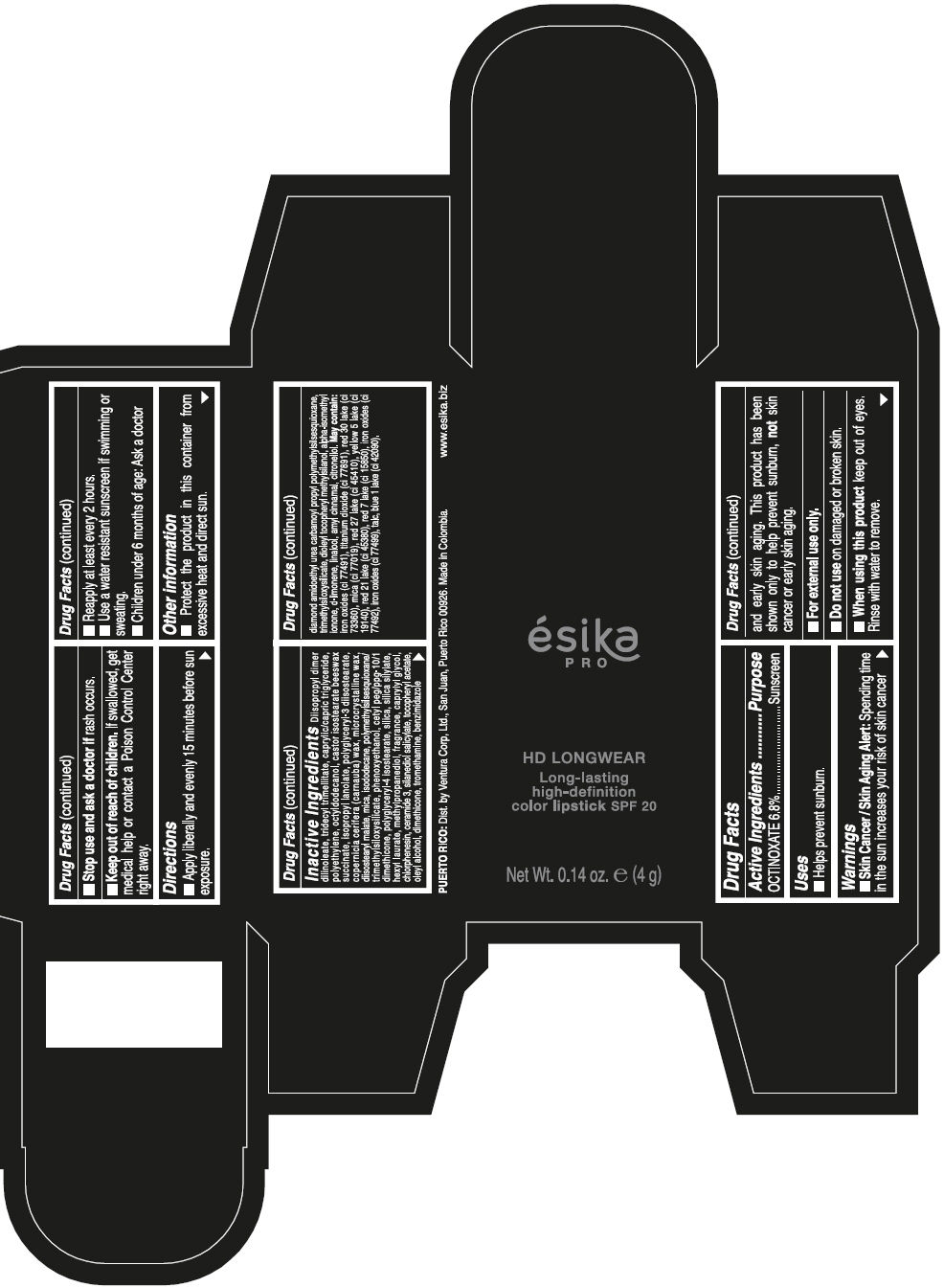
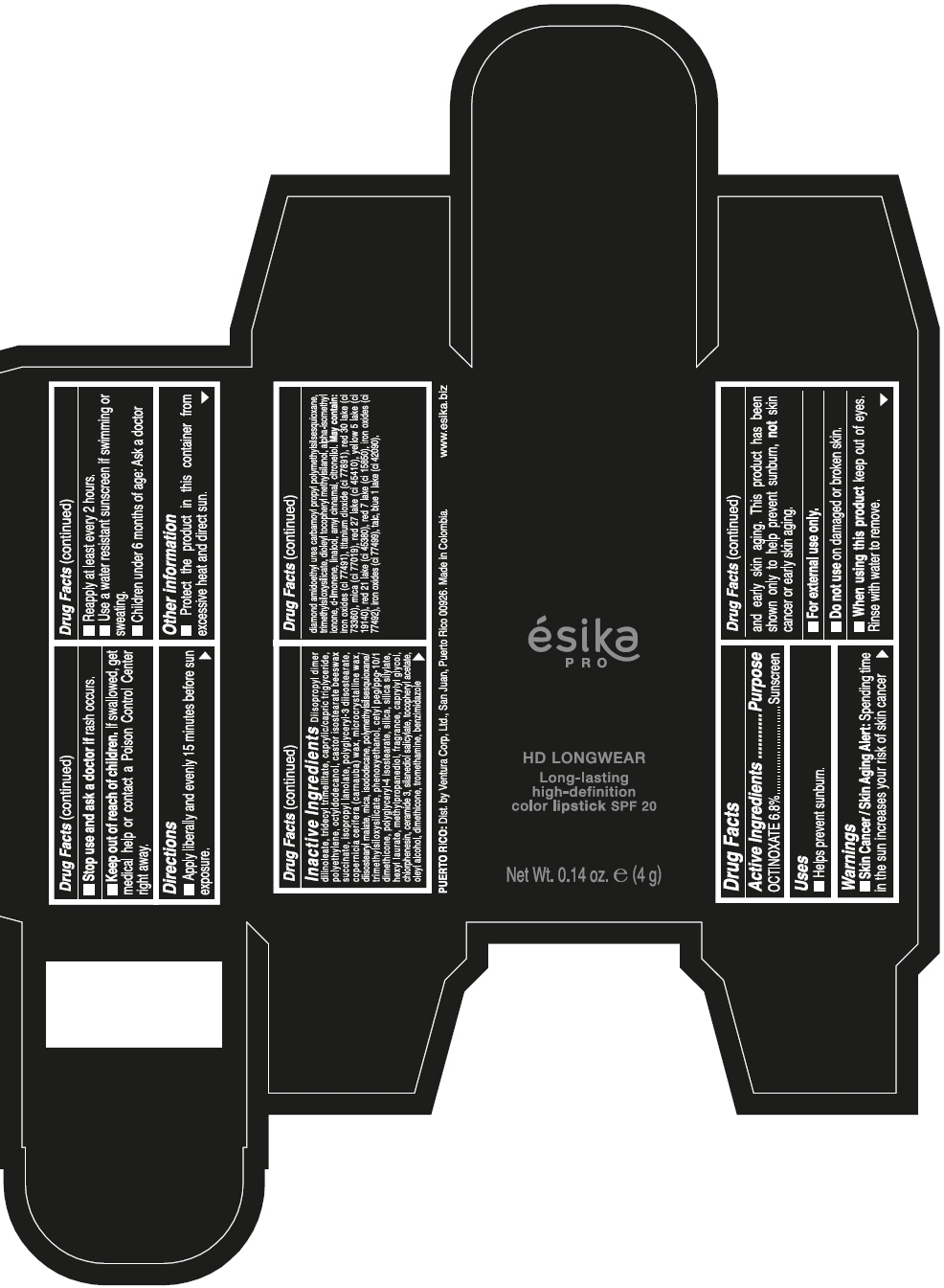
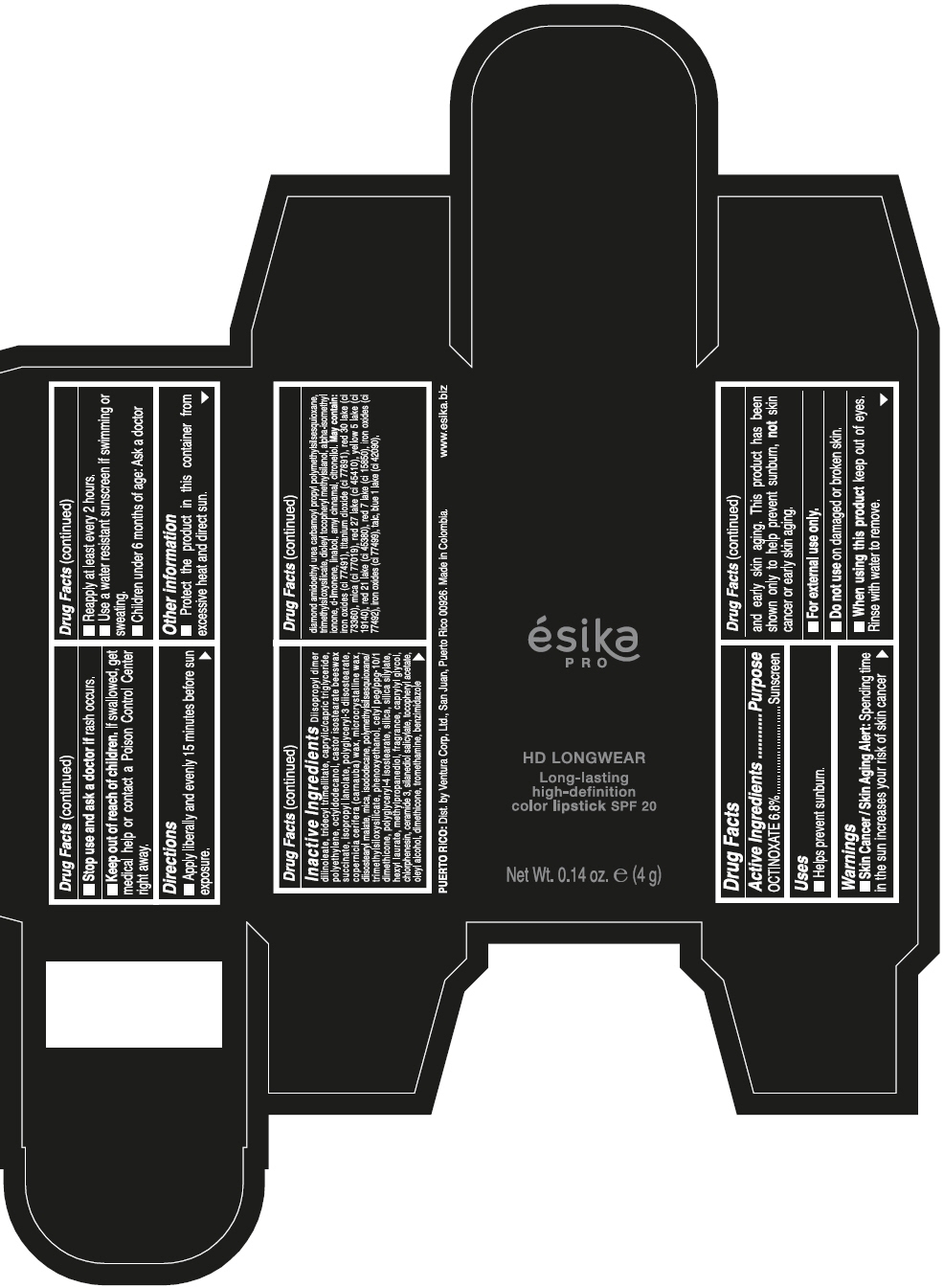
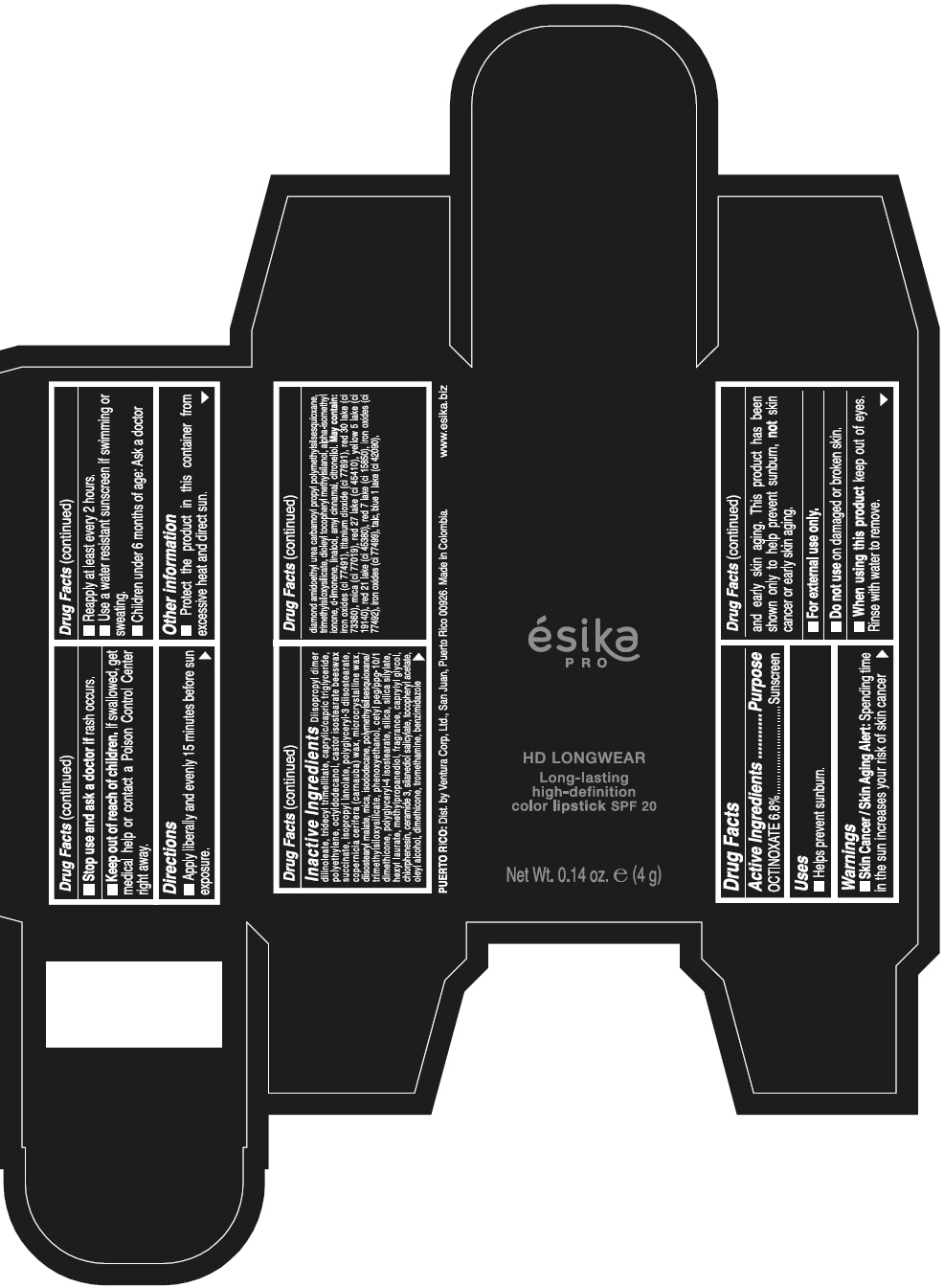
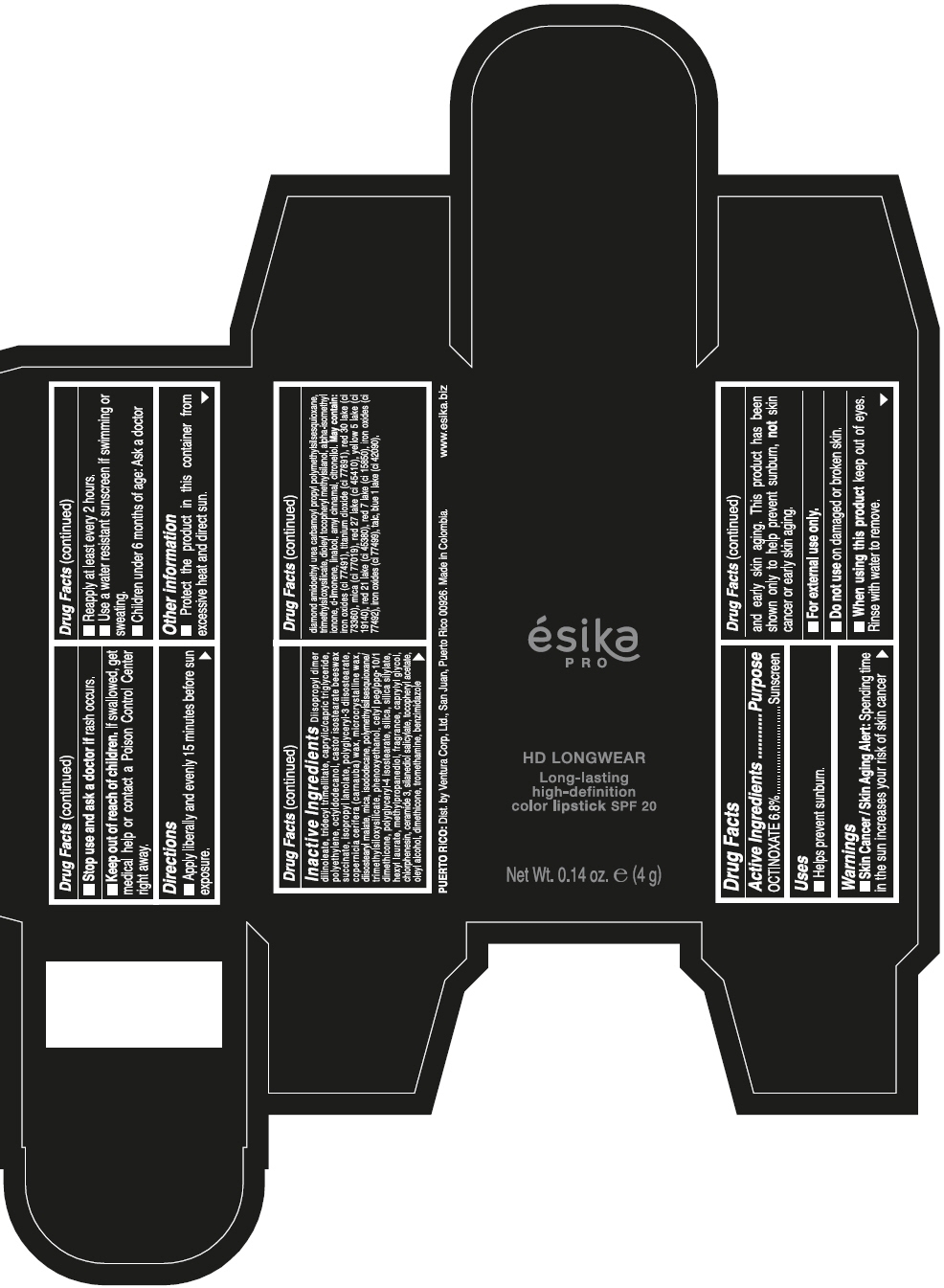
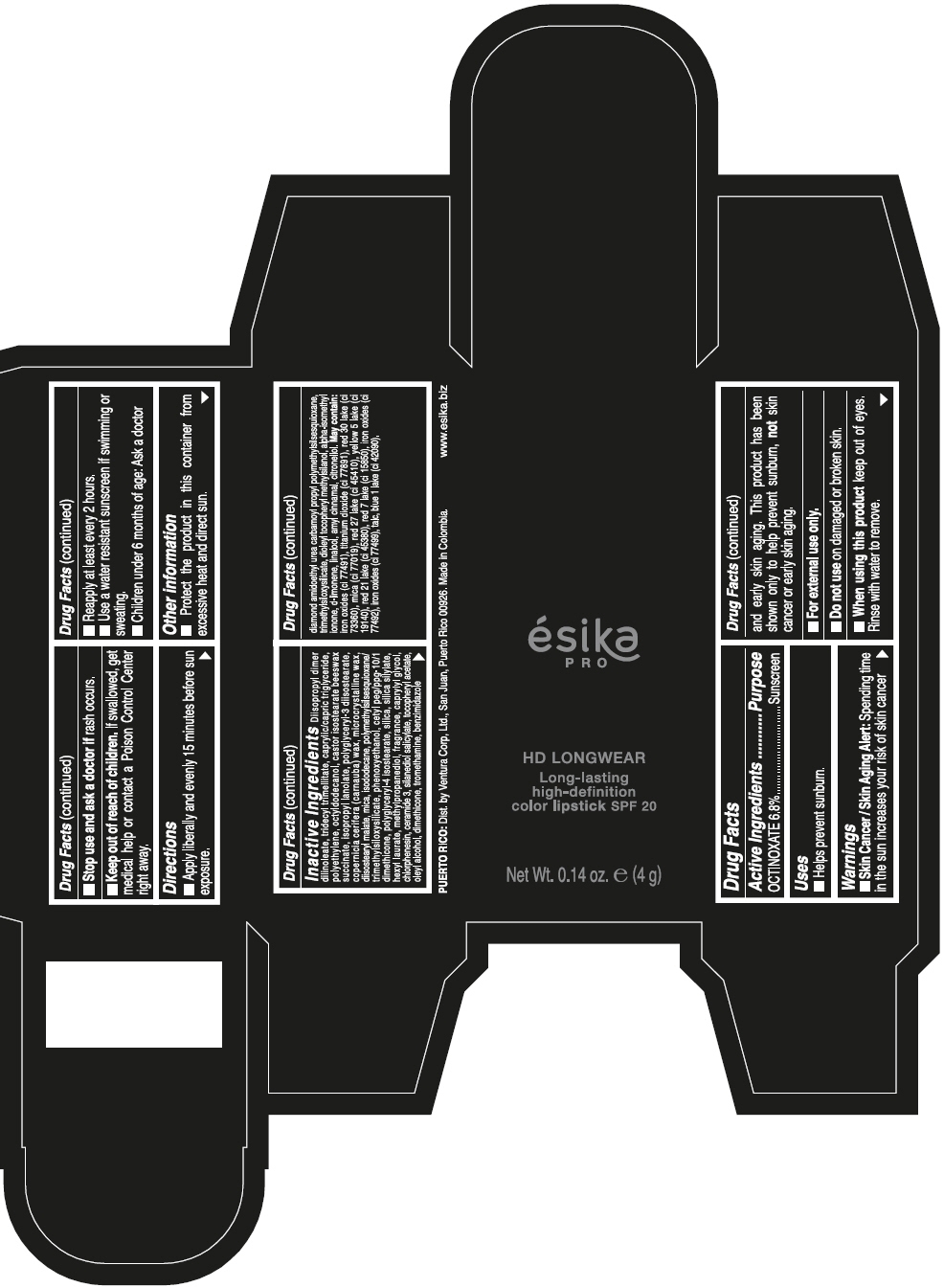
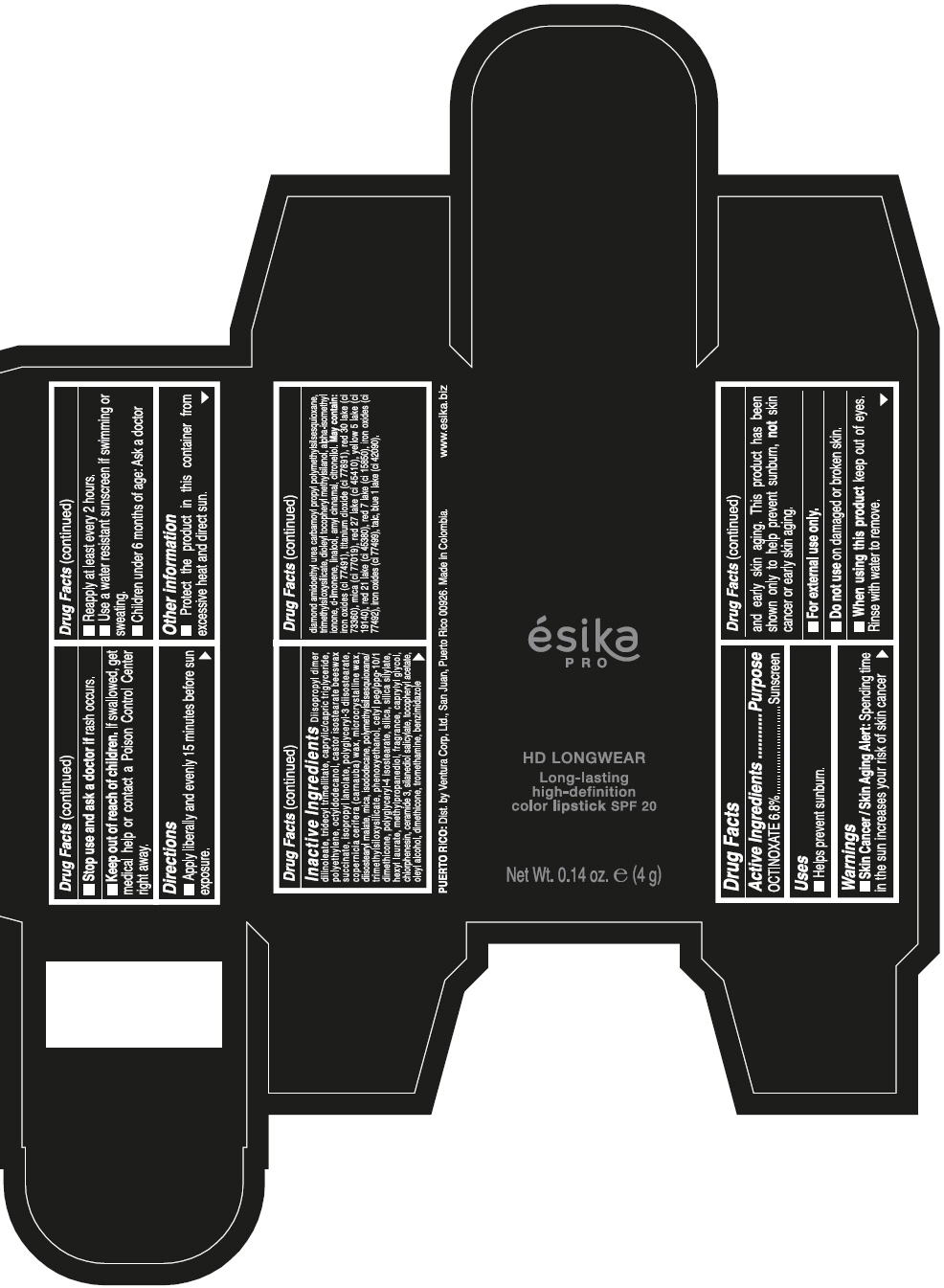
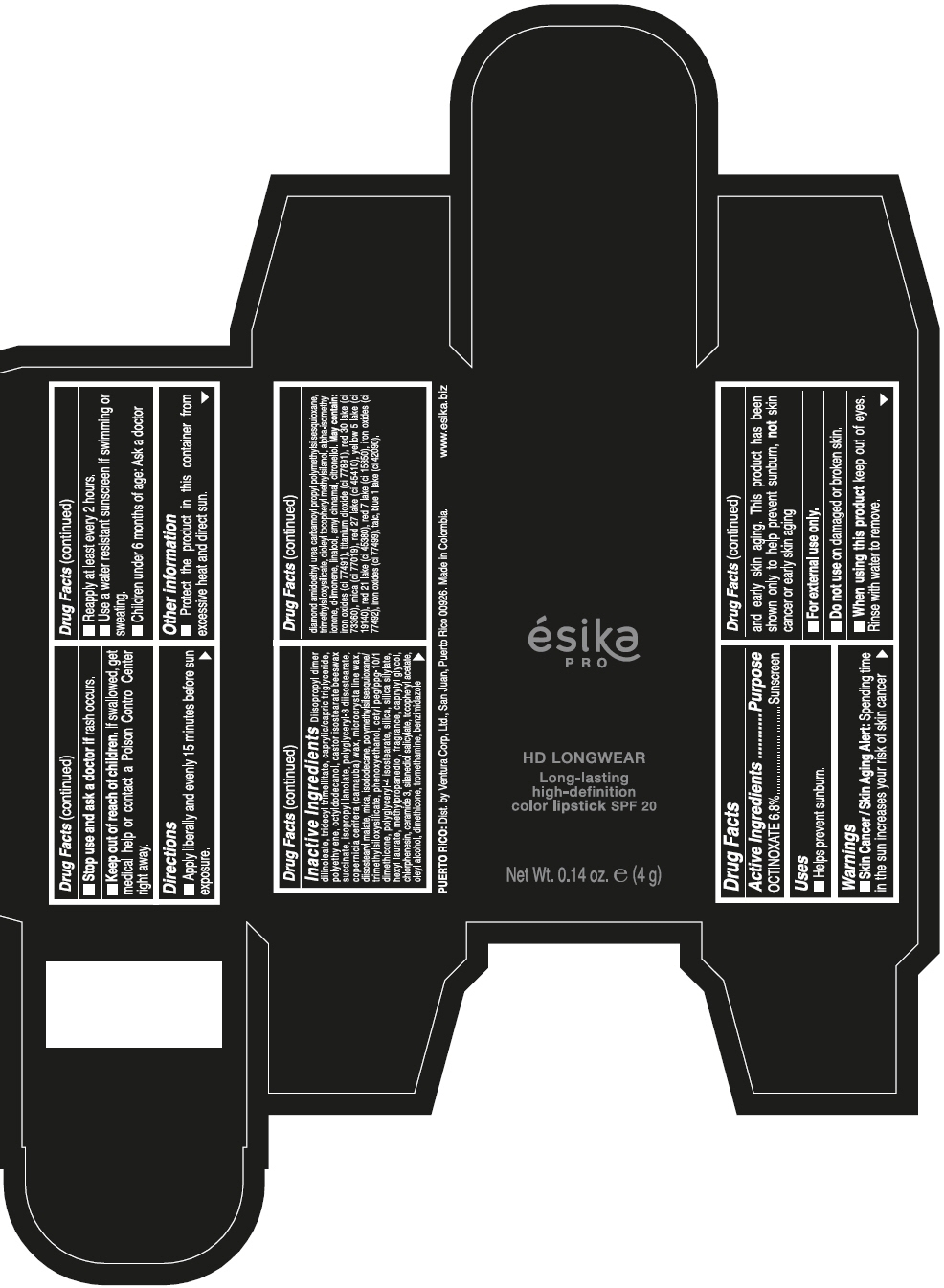
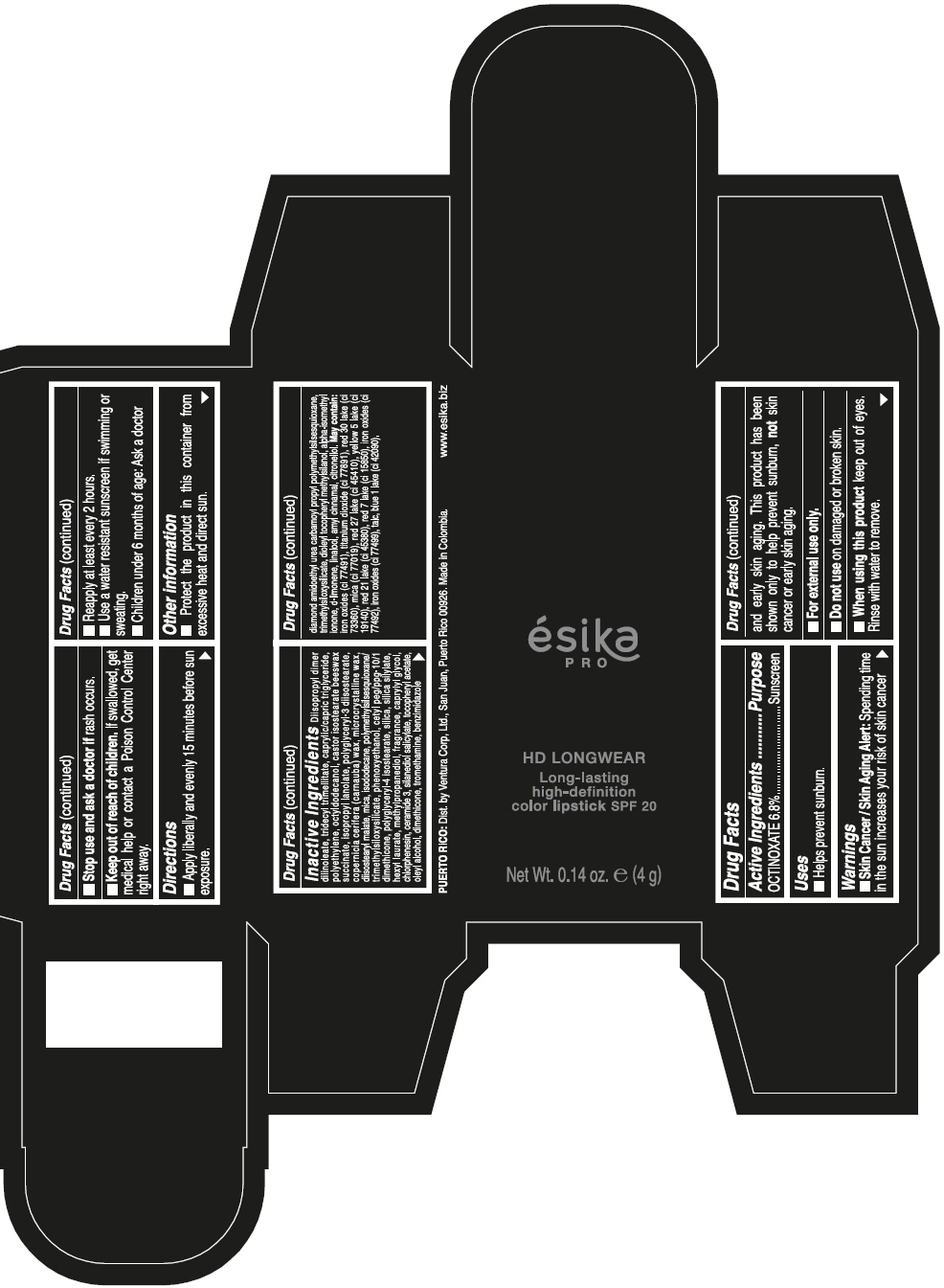
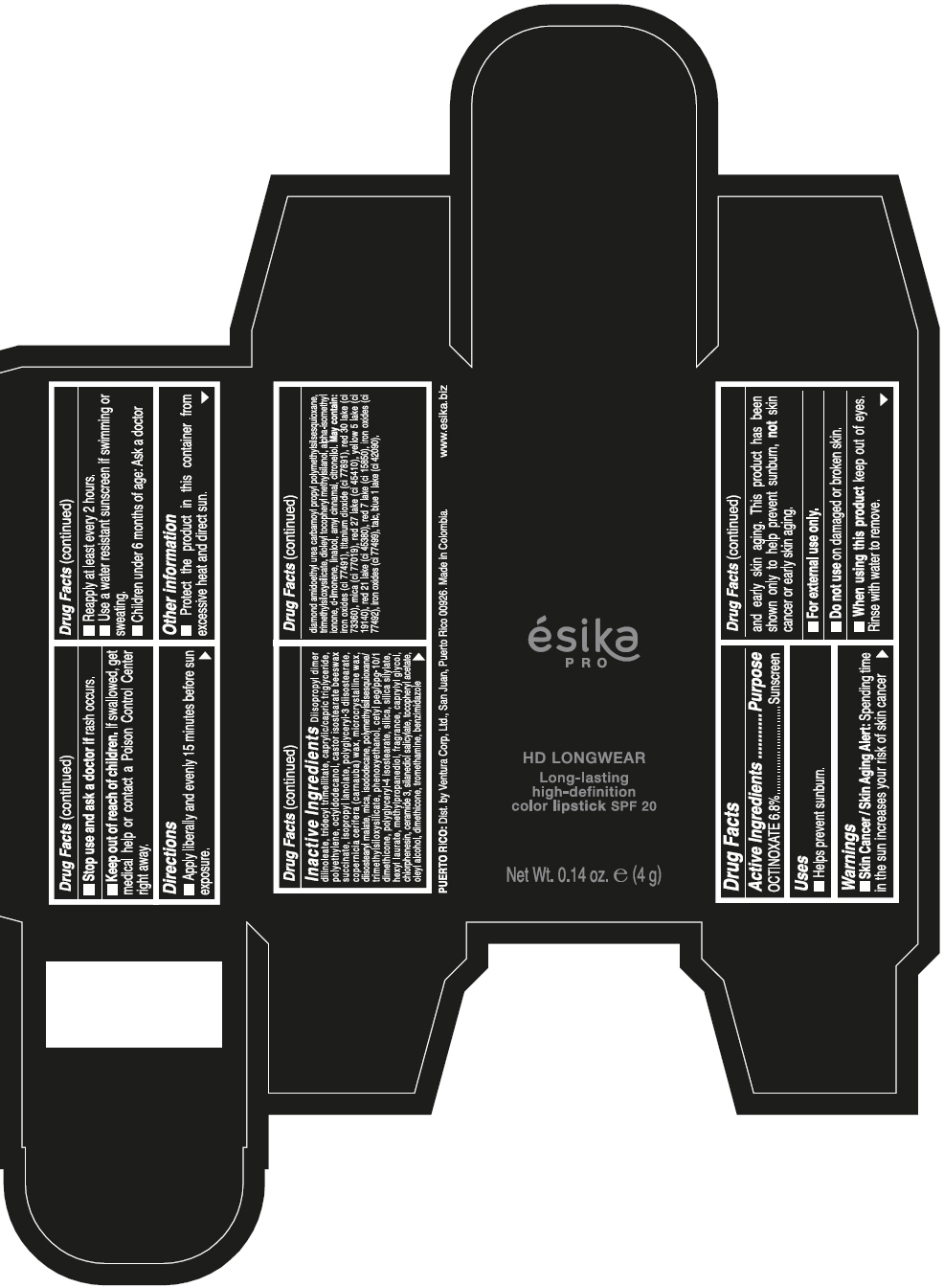
DIISOPROPYL DIMER DILINOLEATE, TRIDECYL TRIMELLITATE, CAPRYLIC/CAPRIC TRIGLYCERIDE, POLYETHYLENE, OCTYLDODECANOL, CASTOR ISOSTEARATE BEESWAX SUCCINATE, ISOPROPYL LANOLATE, POLYGLYCERYL-3 DIISOSTEARATE, COPERNICIA CERIFERA (CARNAUBA) WAX, MICROCRYSTALLINE WAX, DIISOSTEARYL MALATE, MICA, ISODODECANE, POLYMETHYLSILSESQUIOXANE/TRIMETHYLSILOXYSILICATE, PHENOXYETHANOL, CETYL PEG/PPG-10/1 DIMETHICONE, POLYGLYCERYL-4 ISOSTEARATE, SILICA, SILICA SILYLATE, HEXYL LAURATE, METHYLPROPANEDIOL, FRAGRANCE, CAPRYLYL GLYCOL, CHLORPHENESIN, CERAMIDE 3, SILANEDIOL SALICYLATE, TOCOPHERYL ACETATE, OLEYL ALCOHOL, DIMETHICONE, TROMETHAMINE, BENZIMIDAZOLE DIAMOND AMIDOETHYL UREA CARBAMOYL PROPYL POLYMETHYLSILSESQUIOXANE, TRIMETHYLSILOXYSILICATE, DIOLEYL TOCOPHERYL METHYLSILANOL, ALPHA-ISOMETHYL IONONE, d-LIMONENE, LINALOOL, AMYL CINNAMAL, CITRONELLOL. MAY CONTAIN: IRON OXIDES (CI 77491), TITANIUM DIOXIDE (CI 77891), RED 30 LAKE (CI 73360), MICA (CI 77019), RED 27 LAKE (CI 45410), YELLOW 5 LAKE (CI 19140), RED 21 LAKE (CI 45380), RED 7 LAKE (CI 15850), IRON OXIDES (CI 77492), IRON OXIDES (CI 77499), TALC, BLUE 1 LAKE (CI 42090).
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - VINO CAUTIVANTE - PURPLE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - PIMIENTA CALIENTE - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - ROJO GLAM - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - ROJO PASIÓN - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - VINO DESEO - PURPLE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - FUCSIA TENTACIÓN - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - FUCSIA DELIRIO - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - FUCSIA VIBRANTE - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - ROSA VIVA - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - CORAL ENSUEÑO - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - MARRÓN BAMBÚ - BROWN
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - ROSA FIORELLE - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - NATURAL DUNE - BEIGE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - ROJO FIESTA - RED
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - NUDE CREAM - BEIGE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - NUDE MOCCHA - BEIGE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - FUCSIA SUBLIME - PINK
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - MARRÓN HAVANA - BROWN
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - MARRÓN FANATIC - BROWN
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - NUDE ROSE- BEIGE
- PRINCIPAL DISPLAY PANEL - Kit Carton
-
INGREDIENTS AND APPEARANCE
ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 VINO CAUTIVANTE - PURPLE
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43596-0023 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43596-0023-2 1 in 1 BOX 01/27/2017 1 NDC: 43596-0023-1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 PIMIENTA CALIENTE - RED
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43596-0024 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43596-0024-2 1 in 1 BOX 01/27/2017 1 NDC: 43596-0024-1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 ROJO GLAM - RED
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43596-0025 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43596-0025-2 1 in 1 BOX 01/27/2017 1 NDC: 43596-0025-1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 ROJO PASION - RED
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43596-0026 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43596-0026-2 1 in 1 BOX 01/27/2017 1 NDC: 43596-0026-1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 VINO DESEO - PURPLE
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43596-0027 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43596-0027-2 1 in 1 BOX 01/27/2017 1 NDC: 43596-0027-1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 FUCSIA TENTACION - PINK
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43596-0028 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43596-0028-2 1 in 1 BOX 01/27/2017 1 NDC: 43596-0028-1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 FUCSIA DELIRIO - PINK
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43596-0029 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43596-0029-2 1 in 1 BOX 01/27/2017 1 NDC: 43596-0029-1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 FUCSIA VIBRANTE - PINK
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43596-0030 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43596-0030-2 1 in 1 BOX 01/27/2017 1 NDC: 43596-0030-1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 ROSA VIVA - PINK
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43596-0031 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43596-0031-2 1 in 1 BOX 01/27/2017 1 NDC: 43596-0031-1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 CORAL ENSUENO - RED
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43596-0032 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43596-0032-2 1 in 1 BOX 01/27/2017 1 NDC: 43596-0032-1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 MARRON BAMBU - BROWN
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43596-0033 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43596-0033-2 1 in 1 BOX 01/27/2017 1 NDC: 43596-0033-1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 ROSA FIORELLE - PINK
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43596-0034 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43596-0034-2 1 in 1 BOX 01/27/2017 1 NDC: 43596-0034-1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 NATURAL DUNE - BEIGE
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43596-0035 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43596-0035-2 1 in 1 BOX 01/27/2017 1 NDC: 43596-0035-1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 ROJO FIESTA - RED
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43596-0036 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43596-0036-2 1 in 1 BOX 01/27/2017 1 NDC: 43596-0036-1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 NUDE CREAM - BEIGE
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43596-0037 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43596-0037-2 1 in 1 BOX 01/27/2017 1 NDC: 43596-0037-1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 NUDE MOCCHA - BEIGE
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43596-0048 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43596-0048-2 1 in 1 BOX 01/27/2017 1 NDC: 43596-0048-1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 FUCSIA SUBLIME - PINK
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43596-0049 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43596-0049-2 1 in 1 BOX 01/27/2017 1 NDC: 43596-0049-1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 MARRON HAVANA - BROWN
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43596-0050 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43596-0050-2 1 in 1 BOX 01/27/2017 1 NDC: 43596-0050-1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 MARRON FANATIC - BROWN
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43596-0051 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43596-0051-2 1 in 1 BOX 01/27/2017 1 NDC: 43596-0051-1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 NUDE ROSE- BEIGE
octinoxate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43596-0052 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43596-0052-2 1 in 1 BOX 01/27/2017 1 NDC: 43596-0052-1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20
octinoxate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43596-0053 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43596-0053-3 1 in 1 BOX 01/27/2017 1 NDC: 43596-0053-2 12 in 1 TRAY 1 NDC: 43596-0053-1 1 in 1 CONTAINER Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 CONTAINER 0.5833 g Part 2 1 CONTAINER 0.5833 g Part 3 1 CONTAINER 0.5833 g Part 4 1 CONTAINER 0.5833 g Part 5 1 CONTAINER 0.5833 g Part 6 1 CONTAINER 0.5833 g Part 7 1 CONTAINER 0.5833 g Part 8 1 CONTAINER 0.5833 g Part 9 1 CONTAINER 0.5833 g Part 10 1 CONTAINER 0.5833 g Part 11 1 CONTAINER 0.5833 g Part 12 1 CONTAINER 0.5833 g Part 1 of 12 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 VINO CAUTIVANTE - PURPLE
octinoxate pasteProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.5833 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 Part 2 of 12 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 VINO DESEO - PURPLE
octinoxate pasteProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.5833 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 Part 3 of 12 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 MARRON BAMBU - BROWN
octinoxate pasteProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.5833 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 Part 4 of 12 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 NUDE MOCCHA - BEIGE
octinoxate pasteProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.5833 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 Part 5 of 12 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 PIMIENTA CALIENTE - RED
octinoxate pasteProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.5833 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 Part 6 of 12 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 ROJO FIESTA - RED
octinoxate pasteProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.5833 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 Part 7 of 12 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 ROJO PASION - RED
octinoxate pasteProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.5833 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 Part 8 of 12 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 CORAL ENSUENO - RED
octinoxate pasteProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.5833 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 Part 9 of 12 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 FUCSIA TENTACION - PINK
octinoxate pasteProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.5833 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 Part 10 of 12 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 FUCSIA DELIRIO - PINK
octinoxate pasteProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.5833 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 Part 11 of 12 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 NUDE ROSE- BEIGE
octinoxate pasteProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.5833 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 Part 12 of 12 ESIKA PRO HD LONGWEAR LONG-LASTING HIGH-DEFINITION COLOR SPF 20 ROSA FIORELLE - PINK
octinoxate pasteProduct Information Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.068 g in 1 g Inactive Ingredients Ingredient Name Strength DIISOPROPYL DILINOLEATE (UNII: 5323S7S2LR) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) OCTYLDODECANOL (UNII: 461N1O614Y) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) CARNAUBA WAX (UNII: R12CBM0EIZ) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) MICA (UNII: V8A1AW0880) ISODODECANE (UNII: A8289P68Y2) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HEXYL LAURATE (UNII: 4CG9F9W01Q) METHYLPROPANEDIOL (UNII: N8F53B3R4R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CHLORPHENESIN (UNII: I670DAL4SZ) CERAMIDE NP (UNII: 4370DF050B) SILANEDIOL SALICYLATE (UNII: C054DF30K0) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) OLEYL ALCOHOL (UNII: 172F2WN8DV) DIMETHICONE (UNII: 92RU3N3Y1O) TROMETHAMINE (UNII: 023C2WHX2V) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) .ALPHA.-AMYLCINNAMALDEHYDE (UNII: WC51CA3418) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) FERRIC OXIDE RED (UNII: 1K09F3G675) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C RED NO. 30 (UNII: 2S42T2808B) ALUMINUM OXIDE (UNII: LMI26O6933) D&C RED NO. 27 (UNII: 2LRS185U6K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) ACID RED 87 (UNII: TDQ283MPCW) D&C RED NO. 7 (UNII: ECW0LZ41X8) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 0.5833 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/27/2017 Labeler - Ventura Corporation LTD (602751344) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 MANUFACTURE(43596-0023, 43596-0024, 43596-0025, 43596-0026, 43596-0027, 43596-0028, 43596-0029, 43596-0030, 43596-0031, 43596-0032, 43596-0033, 43596-0034, 43596-0035, 43596-0036, 43596-0037, 43596-0048, 43596-0049, 43596-0050, 43596-0051, 43596-0052, 43596-0053)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.