CLETINA hair oil by Guangdong Aimu Biological Technology Co., Ltd
CLETINA hair oil by
Drug Labeling and Warnings
CLETINA hair oil by is a Otc medication manufactured, distributed, or labeled by Guangdong Aimu Biological Technology Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
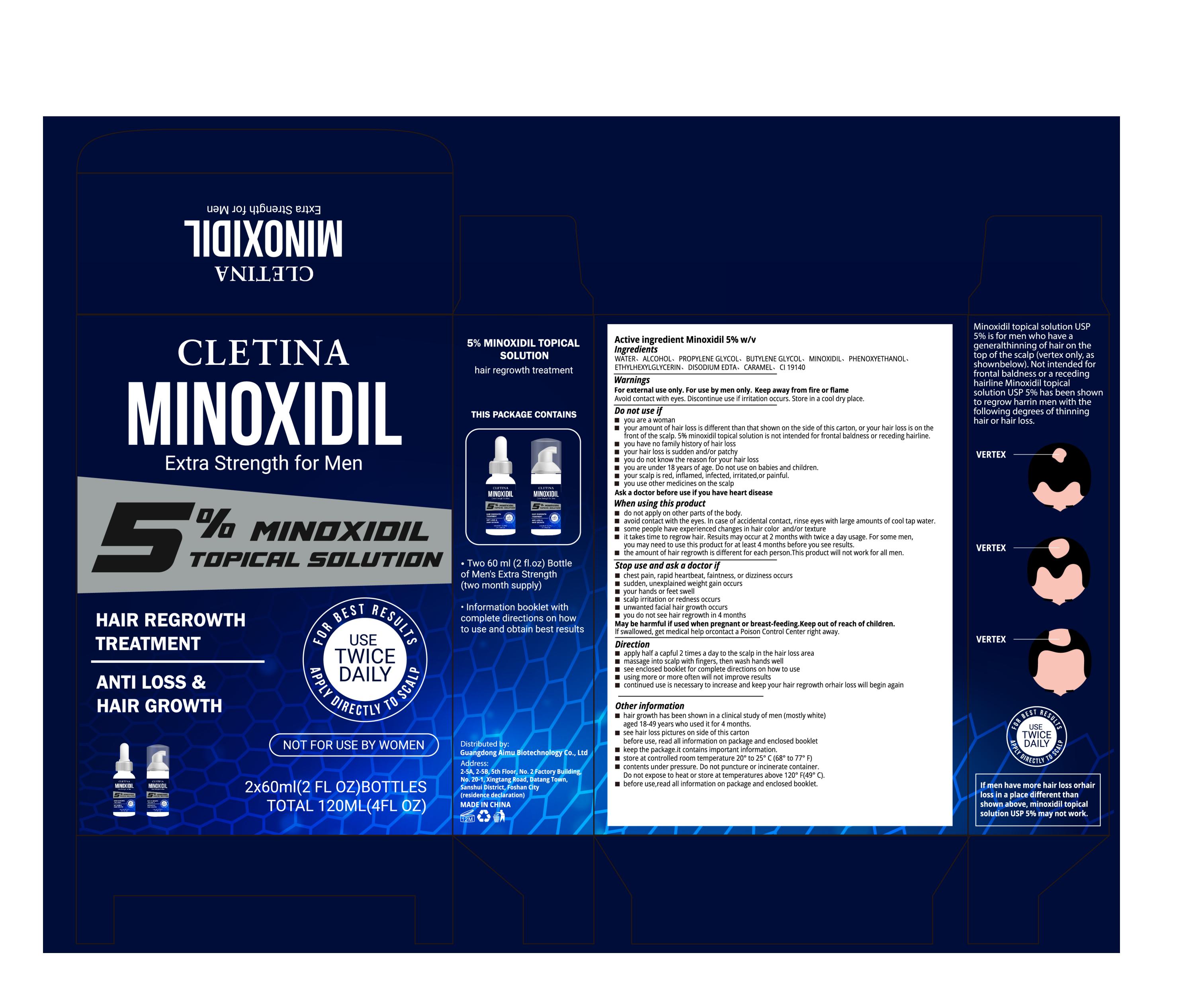
CLETINA HAIR OIL- minoxidil liquid
Guangdong Aimu Biological Technology Co., Ltd
----------
Uses
apply half a capful 2 times a day to the scalp in the hair loss area
massage into scalp with fingers, then wash hands well
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
| CLETINA HAIR OIL
minoxidil liquid |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Guangdong Aimu Biological Technology Co., Ltd (712647107) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Guangdong Aimu Biological Technology Co., Ltd | 712647107 | manufacture(83566-166) | |
Revised: 7/2025
Document Id: 3b328675-27fc-64ed-e063-6394a90a2290
Set id: 1aa99647-3b32-065a-e063-6394a90a9fa1
Version: 2
Effective Time: 20250730
Guang
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.