RETISERT- fluocinolone acetonide implant
Retisert by
Drug Labeling and Warnings
Retisert by is a Prescription medication manufactured, distributed, or labeled by Bausch & Lomb Incorporated, Bausch and Lomb Ireland Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use RETISERT safely and effectively. See full prescribing information for RETISERT.
RETISERT (fluocinolone acetonide intravitreal implant) 0.59 mg for intravitreal use
Initial U.S. Approval: 1963INDICATIONS AND USAGE
RETISERT is a corticosteroid indicated for the treatment of chronic noninfectious uveitis affecting the posterior segment of the eye. (1)
DOSAGE AND ADMINISTRATION
- RETISERT is surgically implanted into the posterior segment of the affected eye through a pars plana incision. (2.1)
- RETISERT is designed to release fluocinolone acetonide at a nominal initial rate of 0.6 mcg/day, decreasing over the first month to a steady state between 0.3-0.4 mcg/day over approximately 30 months. (2.1)
- Aseptic technique should be maintained at all times prior to and during the surgical implantation procedure. (2.2)
DOSAGE FORMS AND STRENGTHS
- 0.59 mg fluocinolone acetonide intravitreal implant. (3)
CONTRAINDICATIONS
- Surgical placement of RETISERT is contraindicated in active viral, bacterial, mycobacterial and fungal infections of ocular structures. (4.1)
WARNINGS AND PRECAUTIONS
- Cataract formation: Nearly all phakic patients are expected to develop cataracts and require cataract surgery. (5.1)
- Endophthalmitis: Late onset endophthalmitis has been observed. (5.2)
- Increase in intraocular pressure: Use of corticosteroids may result in elevated IOP and/or glaucoma. (5.3) IOP lowering medications were required in > 75% of patients; filtering surgeries were required in > 35% of patients. (6.1)
- Separation of implant components: Physicians should periodically monitor the integrity of the implant by visual inspection. (5.4)
ADVERSE REACTIONS
- Ocular adverse events included procedural complications, and eye pain (> 50%). Thirty-five to forty percent of patients reported ocular/conjunctival hyperemia, reduced visual acuity, and conjunctival hemorrhage. (6.1)
- The most common non-ocular event reported was headache (33%). (6.2)
To report SUSPECTED ADVERSE REACTIONS, contact Bausch & Lomb Incorporated at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
2.2 Handling of Implant
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Viral, Bacterial, Mycobacterial and Fungal Infections of Ocular Structures
5 WARNINGS AND PRECAUTIONS
5.1 Cataract Formation
5.2 Endophthalmitis and Surgical Complications
5.3 Increase in Intraocular Pressure
5.4 Separation of Implant Components
5.5 Other Corticosteroid Induced Adverse Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience - Ocular Events
6.2 Clinical Trials Experience - Non-Ocular Events
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information
RETISERT (fluocinolone acetonide intravitreal implant) 0.59 mg is implanted into the posterior segment of the affected eye through a pars plana incision.
The implant contains one tablet of 0.59 mg of fluocinolone acetonide. RETISERT is designed to release fluocinolone acetonide at a nominal initial rate of 0.6 mcg/day, decreasing over the first month to a steady state between 0.3-0.4 mcg/day over approximately 30 months. Following depletion of fluocinolone acetonide as evidenced by recurrence of uveitis, RETISERT may be replaced.
2.2 Handling of Implant
Caution should be exercised in handling RETISERT in order to avoid damage to the implant, which may result in an increased rate of drug release from the implant. Thus, RETISERT should be handled only by the suture tab. Care should be taken during implantation and explantation to avoid sheer forces on the implant that could disengage the silicone cup reservoir (which contains a fluocinolone acetonide tablet) from the suture tab. Aseptic technique should be maintained at all times prior to and during the surgical implantation procedure.
RETISERT should not be resterilized by any method.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Viral, Bacterial, Mycobacterial and Fungal Infections of Ocular Structures
Surgical placement of RETISERT is contraindicated in active viral diseases of the cornea and conjunctiva including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, and varicella, and also in active bacterial, mycobacterial or fungal infections of the eye.
-
5 WARNINGS AND PRECAUTIONS
5.1 Cataract Formation
Use of corticosteroids may result in posterior subcapsular cataract formation.
Based on clinical trials with RETISERT, during the 3-year post-implantation period, nearly all phakic eyes are expected to develop cataracts and require cataract surgery.
5.2 Endophthalmitis and Surgical Complications
Late onset endophthalmitis has been observed. These events are often related to the integrity of the surgical wound site. Careful attention to assure tight closure of the scleral wound and the integrity of the overlying conjunctiva at the wound site is important.
Potential complications accompanying intraocular surgery to place RETISERT into the vitreous cavity may include, but are not limited to, the following: cataract formation, choroidal detachment, endophthalmitis, hypotony, increased intraocular pressure, exacerbation of intraocular inflammation, retinal detachment, vitreous hemorrhage, vitreous loss, and wound dehiscence.
Following implantation of RETISERT, nearly all patients will experience an immediate and temporary decrease in visual acuity in the implanted eye which lasts for approximately one to four weeks post-operatively.
5.3 Increase in Intraocular Pressure
Prolonged use of corticosteroids may result in elevated IOP and/or glaucoma with damage to the optic nerve, defects in visual acuity and fields of vision. Steroids should be used with caution in the presence of glaucoma. Patients must be monitored for elevated IOP.
Based on clinical trials with RETISERT, within 3-years post-implantation, approximately 77% of patients will require IOP lowering medications to control intraocular pressure and 37% of patients will require filtering procedures to control intraocular pressure. [see Adverse Reactions (6.1)].
5.4 Separation of Implant Components
In vitro stability studies show that the strength of the adhesive bond between the silicone cup reservoir and the suture tab is reduced with prolonged hydration, indicating a potential for the separation of these components. The suture tab composition is a silicone elastomer reinforced with a polyester mesh. Physicians should periodically monitor the integrity of the implant by visual inspection.
5.5 Other Corticosteroid Induced Adverse Reactions
RETISERT should be used with caution in patients with a history of a viral, bacterial, mycobacterial or fungal infection of the cornea and conjunctiva including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia and varicella.
Use of ocular steroids may prolong the course and may exacerbate the severity of many viral infections of the eye (including herpes simplex). Employment of a corticosteroid medication in the treatment of patients with a history of herpes simplex requires great caution.
Prolonged use of corticosteroids may suppress the host response and thus increase the hazard of secondary ocular infections (bacterial, fungal, and viral). In acute purulent conditions of the eye, steroids may mask infection or enhance existing infection. Fungal and viral infections of the cornea are particularly prone to develop coincidentally with long-term application of steroids. The possibility of fungal invasion should be considered in any persistent corneal ulceration where steroid treatment has been used.
Since resistance to infections is known to be reduced by corticosteroids, simultaneous bilateral implantation should not be carried out, in order to limit the potential for bilateral post-operative infection.
Ocular administration of corticosteroids has also been associated with delayed wound healing and perforation of the globe where there is thinning of the sclera.
The use of steroids after cataract surgery may delay healing and increase the incidence of bleb formation.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience - Ocular Events
The available safety data includes exposure to RETISERT in patients with chronic non-infectious uveitis affecting the posterior segment in two multicenter controlled clinical trials. Patients were randomized to dosage regimens of 0.59 mg or 2.1 mg implants.
The most frequently reported ocular adverse events were cataract, increased intraocular pressure, procedural complication, and eye pain. These events occurred in approximately 50 - 90% of patients. Cataract includes aggravated cataract, and posterior capsular opacification. Procedural complications includes post-op complication, post-op wound complication, post-op wound site erythema, and wound dehiscense.
Based on clinical trials with RETISERT, during the 3-year post-implantation period, nearly all phakic eyes are expected to develop cataracts and require cataract surgery. IOP lowering medications to lower intraocular pressure were required in approximately 77% of patients; filtering surgeries were required to control intraocular pressure in 37% of patients.
Ocular adverse events occurring in approximately 10 - 40% of patients in decreasing order of incidence were ocular/conjunctival hyperemia, reduced visual acuity, glaucoma, conjunctival hemorrhage, blurred vision, abnormal sensation in the eye, eye irritation, maculopathy, vitreous floaters, hypotony, pruritus, ptosis, increased tearing, vitreous hemorrhage, dry eye, eyelid edema, macular edema and visual disturbance.
Ocular adverse events occurring in approximately 5 - 9% of patients in decreasing order of incidence were eye discharge, photophobia, blepharitis, corneal edema, iris adhesions, choroidal detachment, diplopia, eye swelling, retinal detachment, photopsia, retinal hemorrhage and hyphema.
6.2 Clinical Trials Experience - Non-Ocular Events
The most frequently reported non-ocular adverse event was headache (33%). Other non-ocular adverse events occurring in approximately 5-20% of patients in decreasing order of incidence were nasopharyngitis, arthralgia, sinusitis, dizziness, pyrexia, upper respiratory tract infection, influenza, vomiting, nausea, cough, back pain, limb pain, and rash.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
No adequate animal reproduction studies have been conducted with fluocinolone acetonide.
Corticosteroids are generally teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Fluocinolone acetonide when administered subcutaneously at a dose of 0.13 mg/kg/day (approximately 10,000 times the daily clinical dose of RETISERT), during days 6 to 18 of pregnancy in the rabbit, induced abortion at the end of the third and at the beginning of the fourth gestational week. When administered subcutaneously to rats and rabbits during gestation at a maternal toxic dose of 50 mcg/kg/day (approximately 4,000 times the clinical dose of RETISERT), fluocinolone acetonide caused abortions and malformations in a few surviving fetuses.
There are no adequate and well-controlled studies in pregnant women. RETISERT should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
It is not known whether ocular administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Systemic steroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. Caution should be exercised when RETISERT is implanted in a nursing woman.
-
11 DESCRIPTION
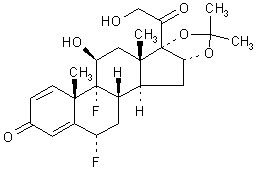
RETISERT® (fluocinolone acetonide intravitreal implant) 0.59 mg is a sterile implant designed to release fluocinolone acetonide locally to the posterior segment of the eye at a nominal initial rate of 0.6 mcg/day, decreasing over the first month to a steady state between 0.3-0.4 mcg/day over approximately 30 months. The drug substance is the synthetic corticosteroid fluocinolone acetonide, represented by the following structural formula:
C24H30F2O6 Mol. Wt. 452.50
Chemical Name: Pregna-1,4-diene-3,20-dione,6,9-difluoro-11,21-dihydroxy-16,17-[(1-methyl-ethylidene)bis(oxy)],(6α,11β ,16α)-.
Fluocinolone acetonide is a white crystalline powder, insoluble in water, and soluble in methanol. It has a melting point of 265-266ºC.
Each RETISERT consists of a tablet containing 0.59 mg of the active ingredient, Fluocinolone Acetonide, USP, and the following inactives: magnesium stearate, microcrystalline cellulose, and polyvinyl alcohol.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Corticosteroids inhibit the inflammatory response to a variety of inciting agents and probably delay or slow healing. They inhibit the edema, fibrin deposition, capillary dilation, leukocyte migration, capillary proliferation, fibroblast proliferation, deposition of collagen, and scar formation associated with inflammation.
There is no generally accepted explanation for the mechanism of action of ocular corticosteroids. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2. Corticosteroids are capable of producing a rise in intraocular pressure.
12.3 Pharmacokinetics
In a subset of patients who received the intravitreal implant, and had blood samples taken at various times (weeks 1, 4 and 34) after implantation, plasma levels of fluocinolone acetonide were below the limit of detection (0.2 ng/mL) at all times. Aqueous and vitreous humor samples were assayed for fluocinolone acetonide in a further subset of patients. While detectable concentrations of fluocinolone acetonide were seen throughout the observation interval (up to 34 months), the concentrations were highly variable, ranging from below the limit of detection (0.2 ng/mL) to 589 ng/mL.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed on RETISERT to evaluate the carcinogenic potential or the effect on fertility of fluocinolone acetonide.
Fluocinolone acetonide was not genotoxic in vitro in the Ames test, the mouse lymphoma TK assay, or in vivo in the mouse bone marrow micronucleus assay.
-
14 CLINICAL STUDIES
In two randomized, double-masked, multicenter controlled clinical trials, 224 patients with chronic (a one year or greater history) non-infectious uveitis affecting the posterior segment of one or both eyes were randomized to receive a 0.59 mg RETISERT. The primary efficacy endpoint in both trials was the rate of recurrence of uveitis affecting the posterior segment of the study eye in the 34 week pre-implantation period compared to the rate of recurrence in the 34 week post-implantation period. Uveitis recurrence rates at 1, 2, and 3 year post-implantation were also compared to the 34 week pre-implantation period.
Detailed results are shown in Table 1 below:
Table 1: Uveitis Recurrence Rates - * Recurrence of uveitis for all post-implantation time points was compared to the 34 weeks pre-implantation time point.
- † p-value <0.01 from McNemar’s χ2 test.
- ‡ Results presented include imputed recurrences. Recurrences were imputed when a subject was not seen within 10 weeks of their final scheduled visit.
TIME POINT
STUDY 1
STUDY 2
N=108
N=116
N (%)
34 Weeks Pre-implantation
58 (53.7)
46 (39.7)
34 Weeks Post-implantation
2 (1.8)
15 (12.9)
1 Year Post-implantation
4 (3.7)
15 (12.9)
2 Years Post-implantation
11 (10.2)
16 (13.8)
3 Years Post-implantation
22 (20.4)
20 (17.2)
3 Years‡ Post-implantation
33 (30.6)
28 (24.1)
-
16 HOW SUPPLIED/STORAGE AND HANDLING
The implant consists of a tablet encased in a silicone elastomer cup containing a release orifice and a polyvinyl alcohol membrane positioned between the tablet and the orifice. The silicone elastomer cup assembly is attached to a silicone elastomer suture tab with silicone adhesive. Each RETISERT is approximately 3 mm x 2 mm x 5 mm.
Each implant is stored in a clear polycarbonate case within a foil pouch within a Tyvek peelable overwrap. Each packaged implant is provided in a carton which includes the package insert.
NDC: 24208-416-01 0.59 mg 1 count
Storage:
Store in the original container at 15°-25°C (59°-77°F).
Protect from freezing. -
17 PATIENT COUNSELING INFORMATION
Patients should be advised to have ophthalmologic follow-up examinations of both eyes at appropriate intervals following implantation of RETISERT.
As with any surgical procedure, there is risk involved. Potential complications accompanying intraocular surgery to place RETISERT into the vitreous cavity may include, but are not limited to, the following: cataract formation, choroidal detachment, temporary decreased visual acuity, endophthalmitis, hypotony, increased intraocular pressure, exacerbation of intraocular inflammation, retinal detachment, vitreous hemorrhage, vitreous loss, and wound dehiscence.
Following implantation of RETISERT, nearly all patients will experience an immediate and temporary decrease in visual acuity in the implanted eye which lasts for approximately one to four weeks post-operatively.
Based on clinical trials with RETISERT, within 3 years post-implantation, approximately 77% of patients will require IOP lowering medications to control intraocular pressure and 37% of patients will require filtering procedures to control intraocular pressure.[see Adverse Reactions (6.1)].
Based on clinical trials with RETISERT, during the 3-year post-implantation period, nearly all phakic eyes are expected to develop cataracts and require cataract surgery.
Manufactured for:
Bausch & Lomb Incorporated
Bridgewater, NJ 08807 USAManufactured by:
Bausch Health Ireland Limited
d/b/a Bausch & Lomb Ireland
Waterford, IrelandRetisert is a trademark of Bausch & Lomb Incorporated or its affiliates.
© 2019 Bausch & Lomb Incorporated or its affiliates9028009
Revised 05/2019
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RETISERT
fluocinolone acetonide implantProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 24208-416 Route of Administration INTRAVITREAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUOCINOLONE ACETONIDE (UNII: 0CD5FD6S2M) (FLUOCINOLONE ACETONIDE - UNII:0CD5FD6S2M) FLUOCINOLONE ACETONIDE 0.59 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 24208-416-01 1 in 1 CARTON 04/08/2005 1 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021737 04/08/2005 Labeler - Bausch & Lomb Incorporated (196603781) Establishment Name Address ID/FEI Business Operations Bausch and Lomb Ireland Limited 986019757 MANUFACTURE(24208-416) , PACK(24208-416) , LABEL(24208-416) Establishment Name Address ID/FEI Business Operations Carton Service Inc. 928861723 LABEL(24208-416)
Trademark Results [Retisert]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RETISERT 78451679 3160088 Live/Registered |
BAUSCH & LOMB INCORPORATED 2004-07-16 |
 RETISERT 78302652 not registered Dead/Abandoned |
BAUSCH & LOMB INCORPORATED 2003-09-19 |
 RETISERT 78024578 not registered Dead/Abandoned |
BAUSCH & LOMB INCORPORATED 2000-09-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.