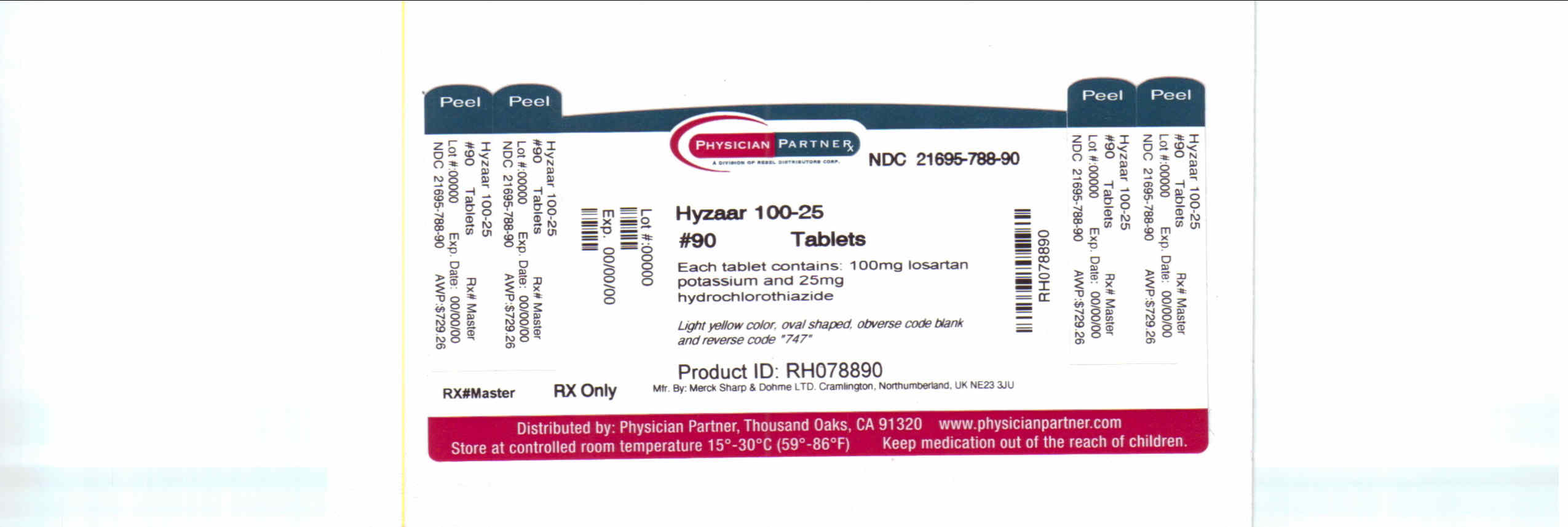
HYZAAR- losartan potassium and hydrochlorothiazide tablet, film coated
HYZAAR by
Drug Labeling and Warnings
HYZAAR by is a Prescription medication manufactured, distributed, or labeled by Rebel Distributors Corp. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
BOXED WARNING
(What is this?)
USE IN PREGNANCY
When used in pregnancy during the second and third trimesters, drugs that act directly on the renin-angiotensin system can cause injury and even death to the developing fetus. When pregnancy is detected, HYZAAR should be discontinued as soon as possible. See WARNINGS, Fetal/Neonatal Morbidity and Mortality.
-
DESCRIPTION
HYZAAR1 50-12.5 (losartan potassium-hydrochlorothiazide), HYZAAR2 100-12.5 (losartan potassium-hydrochlorothiazide) and HYZAAR3 100-25 (losartan potassium-hydrochlorothiazide) combine an angiotensin II receptor (type AT1) antagonist and a diuretic, hydrochlorothiazide.
Losartan potassium, a non-peptide molecule, is chemically described as 2-butyl-4-chloro-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]imidazole-5-methanol monopotassium salt. Its empirical formula is C22H22ClKN6O, and its structural formula is:
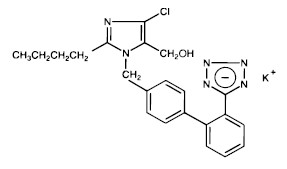
Losartan potassium is a white to off-white free-flowing crystalline powder with a molecular weight of 461.01. It is freely soluble in water, soluble in alcohols, and slightly soluble in common organic solvents, such as acetonitrile and methyl ethyl ketone.
Oxidation of the 5-hydroxymethyl group on the imidazole ring results in the active metabolite of losartan.
Hydrochlorothiazide is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2 and its structural formula is:
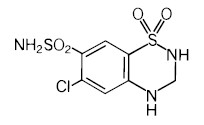
Hydrochlorothiazide is a white, or practically white, crystalline powder with a molecular weight of 297.74, which is slightly soluble in water, but freely soluble in sodium hydroxide solution.
HYZAAR is available for oral administration in three tablet combinations of losartan and hydrochlorothiazide. HYZAAR 50-12.5 contains 50 mg of losartan potassium and 12.5 mg of hydrochlorothiazide. HYZAAR 100-12.5 contains 100 mg of losartan potassium and 12.5 mg of hydrochlorothiazide. HYZAAR 100-25 contains 100 mg of losartan potassium and 25 mg of hydrochlorothiazide. Inactive ingredients are microcrystalline cellulose, lactose hydrous, pregelatinized starch, magnesium stearate, hydroxypropyl cellulose, hypromellose, and titanium dioxide. HYZAAR 50-12.5 and HYZAAR 100-25 also contain D&C yellow No. 10 aluminum lake. HYZAAR 50-12.5, HYZAAR 100-12.5, and HYZAAR 100-25 may also contain carnauba wax.
HYZAAR 50-12.5 contains 4.24 mg (0.108 mEq) of potassium, HYZAAR 100-12.5 contains 8.48 mg (0.216 mEq) of potassium, and HYZAAR 100-25 contains 8.48 mg (0.216 mEq) of potassium.
- 1
Registered trademark of E.I. du Pont de Nemours and Company, Wilmington, Delaware, USA
Copyright © 1995, 2005 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved- 2
Registered trademark of E.I. du Pont de Nemours and Company, Wilmington, Delaware, USA
Copyright © 1995, 2005 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved- 3
Registered trademark of E.I. du Pont de Nemours and Company, Wilmington, Delaware, USA
Copyright © 1995, 2005 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reserved - 1
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Angiotensin II [formed from angiotensin I in a reaction catalyzed by angiotensin converting enzyme (ACE, kininase II)], is a potent vasoconstrictor, the primary vasoactive hormone of the renin-angiotensin system and an important component in the pathophysiology of hypertension. It also stimulates aldosterone secretion by the adrenal cortex. Losartan and its principal active metabolite block the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor found in many tissues (e.g., vascular smooth muscle, adrenal gland). There is also an AT2 receptor found in many tissues but it is not known to be associated with cardiovascular homeostasis. Both losartan and its principal active metabolite do not exhibit any partial agonist activity at the AT1 receptor and have much greater affinity (about 1000-fold) for the AT1 receptor than for the AT2 receptor. In vitro binding studies indicate that losartan is a reversible, competitive inhibitor of the AT1 receptor. The active metabolite is 10 to 40 times more potent by weight than losartan and appears to be a reversible, non-competitive inhibitor of the AT1 receptor.
Neither losartan nor its active metabolite inhibits ACE (kininase II, the enzyme that converts angiotensin I to angiotensin II and degrades bradykinin); nor do they bind to or block other hormone receptors or ion channels known to be important in cardiovascular regulation.
Hydrochlorothiazide is a thiazide diuretic. Thiazides affect the renal tubular mechanisms of electrolyte reabsorption, directly increasing excretion of sodium and chloride in approximately equivalent amounts. Indirectly, the diuretic action of hydrochlorothiazide reduces plasma volume, with consequent increases in plasma renin activity, increases in aldosterone secretion, increases in urinary potassium loss, and decreases in serum potassium. The renin-aldosterone link is mediated by angiotensin II, so coadministration of an angiotensin II receptor antagonist tends to reverse the potassium loss associated with these diuretics.
The mechanism of the antihypertensive effect of thiazides is unknown.
Pharmacokinetics
General
Losartan Potassium
Losartan is an orally active agent that undergoes substantial first-pass metabolism by cytochrome P450 enzymes. It is converted, in part, to an active carboxylic acid metabolite that is responsible for most of the angiotensin II receptor antagonism that follows losartan treatment. The terminal half-life of losartan is about 2 hours and of the metabolite is about 6-9 hours. The pharmacokinetics of losartan and its active metabolite are linear with oral losartan doses up to 200 mg and do not change over time. Neither losartan nor its metabolite accumulate in plasma upon repeated once-daily dosing.
Following oral administration, losartan is well absorbed (based on absorption of radiolabeled losartan) and undergoes substantial first-pass metabolism; the systemic bioavailability of losartan is approximately 33%. About 14% of an orally-administered dose of losartan is converted to the active metabolite. Mean peak concentrations of losartan and its active metabolite are reached in 1 hour and in 3-4 hours, respectively. While maximum plasma concentrations of losartan and its active metabolite are approximately equal, the AUC of the metabolite is about 4 times as great as that of losartan. A meal slows absorption of losartan and decreases its Cmax but has only minor effects on losartan AUC or on the AUC of the metabolite (about 10% decreased).
Both losartan and its active metabolite are highly bound to plasma proteins, primarily albumin, with plasma free fractions of 1.3% and 0.2%, respectively. Plasma protein binding is constant over the concentration range achieved with recommended doses. Studies in rats indicate that losartan crosses the blood-brain barrier poorly, if at all.
Losartan metabolites have been identified in human plasma and urine. In addition to the active carboxylic acid metabolite, several inactive metabolites are formed. Following oral and intravenous administration of 14C-labeled losartan potassium, circulating plasma radioactivity is primarily attributed to losartan and its active metabolite. In vitro studies indicate that cytochrome P450 2C9 and 3A4 are involved in the biotransformation of losartan to its metabolites. Minimal conversion of losartan to the active metabolite (less than 1% of the dose compared to 14% of the dose in normal subjects) was seen in about one percent of individuals studied.
The volume of distribution of losartan is about 34 liters and of the active metabolite is about 12 liters. Total plasma clearance of losartan and the active metabolite is about 600 mL/min and 50 mL/min, respectively, with renal clearance of about 75 mL/min and 25 mL/min, respectively. When losartan is administered orally, about 4% of the dose is excreted unchanged in the urine and about 6% is excreted in urine as active metabolite. Biliary excretion contributes to the elimination of losartan and its metabolites. Following oral 14C-labeled losartan, about 35% of radioactivity is recovered in the urine and about 60% in the feces. Following an intravenous dose of 14C-labeled losartan, about 45% of radioactivity is recovered in the urine and 50% in the feces.
Special Populations
Pediatric: Losartan pharmacokinetics have been investigated in patients 6 to 16 years (see PRECAUTIONS, Pediatric Use).
Geriatric and Gender: Losartan pharmacokinetics have been investigated in the elderly (65-75 years) and in both genders. Plasma concentrations of losartan and its active metabolite are similar in elderly and young hypertensives. Plasma concentrations of losartan were about twice as high in female hypertensives as male hypertensives, but concentrations of the active metabolite were similar in males and females.
Race: Pharmacokinetic differences due to race have not been studied (see also PRECAUTIONS, Race and CLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical Effects, Losartan Potassium, Reduction in the Risk of Stroke, Race).
Renal Insufficiency:
Losartan: Following oral administration, plasma concentrations and AUCs of losartan and its active metabolite are increased by 50-90% in patients with mild (creatinine clearance of 50 to 74 mL/min) or moderate (creatinine clearance 30 to 49 mL/min) renal insufficiency. In this study, renal clearance was reduced by 55-85% for both losartan and its active metabolite in patients with mild or moderate renal insufficiency. Neither losartan nor its active metabolite can be removed by hemodialysis.
Hydrochlorothiazide: Following oral administration, the AUC for hydrochlorothiazide is increased by 70 and 700% for patients with mild and moderate renal insufficiency, respectively. In this study, renal clearance of hydrochlorothiazide decreased by 45 and 85% in patients with mild and moderate renal impairment, respectively.
The usual regimens of therapy with HYZAAR may be followed as long as the patient's creatinine clearance is >30 mL/min. In patients with more severe renal impairment, loop diuretics are preferred to thiazides, so HYZAAR is not recommended. (See DOSAGE AND ADMINISTRATION.)
Hepatic Insufficiency: Following oral administration in patients with mild to moderate alcoholic cirrhosis of the liver, plasma concentrations of losartan and its active metabolite were, respectively, 5 times and about 1.7 times those in young male volunteers. Compared to normal subjects, the total plasma clearance of losartan in patients with hepatic insufficiency was about 50% lower, and the oral bioavailability was about 2 times higher. The lower starting dose of losartan recommended for use in patients with hepatic impairment cannot be given using HYZAAR. Its use in such patients as a means of losartan titration is, therefore, not recommended (see DOSAGE AND ADMINISTRATION).
Drug Interactions
Losartan Potassium
Losartan, administered for 12 days, did not affect the pharmacokinetics or pharmacodynamics of a single dose of warfarin. Losartan did not affect the pharmacokinetics of oral or intravenous digoxin. There is no pharmacokinetic interaction between losartan and hydrochlorothiazide. Coadministration of losartan and cimetidine led to an increase of about 18% in AUC of losartan but did not affect the pharmacokinetics of its active metabolite. Coadministration of losartan and phenobarbital led to a reduction of about 20% in the AUC of losartan and that of its active metabolite. A somewhat greater interaction (approximately 40% reduction in the AUC of active metabolite and approximately 30% reduction in the AUC of losartan) has been reported with rifampin. Fluconazole, an inhibitor of cytochrome P450 2C9, decreased the AUC of the active metabolite by approximately 40%, but increased the AUC of losartan by approximately 70% following multiple doses. Conversion of losartan to its active metabolite after intravenous administration is not affected by ketoconazole, an inhibitor of P450 3A4. The AUC of active metabolite following oral losartan was not affected by erythromycin, another inhibitor of P450 3A4, but the AUC of losartan was increased by 30%.
Hydrochlorothiazide
After oral administration of hydrochlorothiazide, diuresis begins within 2 hours, peaks in about 4 hours and lasts about 6 to 12 hours.
Hydrochlorothiazide is not metabolized but is eliminated rapidly by the kidney. When plasma levels have been followed for at least 24 hours, the plasma half-life has been observed to vary between 5.6 and 14.8 hours. At least 61 percent of the oral dose is eliminated unchanged within 24 hours. Hydrochlorothiazide crosses the placental but not the blood-brain barrier and is excreted in breast milk.
Pharmacodynamics and Clinical Effects
Losartan Potassium
Hypertension
Losartan inhibits the pressor effect of angiotensin II (as well as angiotensin I) infusions. A dose of 100 mg inhibits the pressor effect by about 85% at peak with 25-40% inhibition persisting for 24 hours. Removal of the negative feedback of angiotensin II causes a 2- to 3-fold rise in plasma renin activity and consequent rise in angiotensin II plasma concentration in hypertensive patients. Losartan does not affect the response to bradykinin, whereas ACE inhibitors increase the response to bradykinin. Aldosterone plasma concentrations fall following losartan administration. In spite of the effect of losartan on aldosterone secretion, very little effect on serum potassium was observed.
In a single-dose study in normal volunteers, losartan had no effects on glomerular filtration rate, renal plasma flow or filtration fraction. In multiple-dose studies in hypertensive patients, there were no notable effects on systemic or renal prostaglandin concentrations, fasting triglycerides, total cholesterol or HDL-cholesterol or fasting glucose concentrations. There was a small uricosuric effect leading to a minimal decrease in serum uric acid (mean decrease <0.4 mg/dL) during chronic oral administration.
The antihypertensive effects of losartan were demonstrated principally in 4 placebo-controlled, 6- to 12-week trials of dosages from 10 to 150 mg per day in patients with baseline diastolic blood pressures of 95-115. The studies allowed comparisons of two doses (50-100 mg/day) as once-daily or twice-daily regimens, comparisons of peak and trough effects, and comparisons of response by gender, age, and race. Three additional studies examined the antihypertensive effects of losartan and hydrochlorothiazide in combination.
The 4 studies of losartan monotherapy included a total of 1075 patients randomized to several doses of losartan and 334 to placebo. The 10 and 25 mg doses produced some effect at peak (6 hours after dosing) but small and inconsistent trough (24 hour) responses. Doses of 50, 100, and 150 mg once daily gave statistically significant systolic/diastolic mean decreases in blood pressure, compared to placebo in the range of 5.5-10.5/3.5-7.5 mmHg, with the 150 mg dose giving no greater effect than 50-100 mg. Twice-daily dosing at 50-100 mg/day gave consistently larger trough responses than once-daily dosing at the same total dose. Peak (6 hour) effects were uniformly, but moderately larger than trough effects, with the trough to peak ratio for systolic and diastolic responses 50-95% and 60-90%, respectively.
Analysis of age, gender, and race subgroups of patients showed that men and women, and patients over and under 65, had generally similar responses. Losartan was effective in reducing blood pressure regardless of race, although the effect was somewhat less in Black patients (usually a low-renin population).
The effect of losartan is substantially present within one week but in some studies the maximal effect occurred in 3-6 weeks. In long-term follow-up studies (without placebo control) the effect of losartan appeared to be maintained for up to a year. There is no apparent rebound effect after abrupt withdrawal of losartan. There was essentially no change in average heart rate in losartan-treated patients in controlled trials.
Reduction in the Risk of Stroke
The Losartan Intervention For Endpoint reduction in hypertension (LIFE) study was a multinational, double-blind study comparing losartan and atenolol in 9193 hypertensive patients with ECG-documented left ventricular hypertrophy. Patients with myocardial infarction or stroke within six months prior to randomization were excluded. Patients were randomized to receive once daily losartan 50 mg or atenolol 50 mg. If goal blood pressure (<140/90 mmHg) was not reached, hydrochlorothiazide (12.5 mg) was added first and, if needed, the dose of losartan or atenolol was then increased to 100 mg once daily. If necessary, other antihypertensive treatments (e.g., increase in dose of hydrochlorothiazide therapy to 25 mg or addition of other diuretic therapy, calcium channel blockers, alpha-blockers, or centrally acting agents, but not ACE inhibitors, angiotensin II antagonists, or beta-blockers) were added to the treatment regimen to reach the goal blood pressure.
In efforts to control blood pressure, the patients in both arms of the LIFE study were coadministered hydrochlorothiazide the majority of time they were on study drug (73.9% and 72.4% of days in the losartan and atenolol arms, respectively).
Of the randomized patients, 4963 (54%) were female and 533 (6%) were Black. The mean age was 67 with 5704 (62%) age ≥65. At baseline, 1195 (13%) had diabetes, 1326 (14%) had isolated systolic hypertension, 1469 (16%) had coronary heart disease, and 728 (8%) had cerebrovascular disease. Baseline mean blood pressure was 174/98 mmHg in both treatment groups. The mean length of follow-up was 4.8 years. At the end of study or at the last visit before a primary endpoint, 77% of the group treated with losartan and 73% of the group treated with atenolol were still taking study medication. Of the patients still taking study medication, the mean doses of losartan and atenolol were both about 80 mg/day, and 15% were taking atenolol or losartan as monotherapy, while 77% were also receiving hydrochlorothiazide (at a mean dose of 20 mg/day in each group). Blood pressure reduction measured at trough was similar for both treatment groups but blood pressure was not measured at any other time of the day. At the end of study or at the last visit before a primary endpoint, the mean blood pressures were 144.1/81.3 mmHg for the group treated with losartan and 145.4/80.9 mmHg for the group treated with atenolol [the difference in SBP of 1.3 mmHg was significant (p<0.001), while the difference of 0.4 mmHg in DBP was not significant (p=0.098)].
The primary endpoint was the first occurrence of cardiovascular death, nonfatal stroke, or nonfatal myocardial infarction. Patients with nonfatal events remained in the trial, so that there was also an examination of the first event of each type even if it was not the first event (e.g., a stroke following an initial myocardial infarction would be counted in the analysis of stroke). Treatment with losartan resulted in a 13% reduction (p=0.021) in risk of the primary endpoint compared to the atenolol group; this difference was primarily the result of an effect on fatal and nonfatal stroke. Treatment with losartan reduced the risk of stroke by 25% relative to atenolol (p=0.001).
For additional details on the LIFE study see the label for COZAAR.
Race
In the LIFE study, Black patients treated with atenolol were at lower risk of experiencing the primary composite endpoint compared with Black patients treated with losartan. In the subgroup of Black patients (n=533, 6% of the LIFE study patients), there were 29 primary endpoints among 263 patients on atenolol (11%, 26 per 1000 patient-years) and 46 primary endpoints among 270 patients (17%, 42 per 1000 patient-years) on losartan. This finding could not be explained on the basis of differences in the populations other than race or on any imbalances between treatment groups. In addition, blood pressure reductions in both treatment groups were consistent between Black and non-Black patients. Given the difficulty in interpreting subset differences in large trials, it cannot be known whether the observed difference is the result of chance. However, the LIFE study provides no evidence that the benefits of losartan on reducing the risk of cardiovascular events in hypertensive patients with left ventricular hypertrophy apply to Black patients.
Losartan Potassium-Hydrochlorothiazide
The 3 controlled studies of losartan and hydrochlorothiazide included over 1300 patients assessing the antihypertensive efficacy of various doses of losartan (25, 50 and 100 mg) and concomitant hydrochlorothiazide (6.25, 12.5 and 25 mg). A factorial study compared the combination of losartan/hydrochlorothiazide 50/12.5 mg with its components and placebo. The combination of losartan/hydrochlorothiazide 50/12.5 mg resulted in an approximately additive placebo-adjusted systolic/diastolic response (15.5/9.0 mmHg for the combination compared to 8.5/5.0 mmHg for losartan alone and 7.0/3.0 mmHg for hydrochlorothiazide alone). Another study investigated the dose-response relationship of various doses of hydrochlorothiazide (6.25, 12.5 and 25 mg) or placebo on a background of losartan (50 mg) in patients not adequately controlled (sitting diastolic blood pressure [SiDBP] 93-120 mmHg) on losartan (50 mg) alone. The third study investigated the dose-response relationship of various doses of losartan (25, 50 and 100 mg) or placebo on a background of hydrochlorothiazide (25 mg) in patients not adequately controlled (SiDBP 93-120 mmHg) on hydrochlorothiazide (25 mg) alone. These studies showed an added antihypertensive response at trough (24 hours post-dosing) of hydrochlorothiazide 12.5 or 25 mg added to losartan 50 mg of 5.5/3.5 and 10.0/6.0 mmHg, respectively. Similarly, there was an added antihypertensive response at trough when losartan 50 or 100 mg was added to hydrochlorothiazide 25 mg of 9.0/5.5 and 12.5/6.5 mmHg, respectively. There was no significant effect on heart rate.
There was no difference in response for men and women or in patients over or under 65 years of age.
Black patients had a larger response to hydrochlorothiazide than non-Black patients and a smaller response to losartan. The overall response to the combination was similar for Black and non-Black patients.
Severe Hypertension (Sitting Diastolic Blood Pressure [SiDBP]≥110 mmHg)
The safety and efficacy of HYZAAR as initial therapy for severe hypertension (defined as a mean SiDBP ≥110 mmHg confirmed on 2 separate occasions off all antihypertensive therapy) was studied in a 6-week double-blind, randomized, multicenter study. Patients were randomized to either losartan and hydrochlorothiazide (50-12.5 mg, once daily) or to losartan (50 mg, once daily) and followed for blood pressure response. Patients were titrated at 2-week intervals if their SiDBP did not reach goal (<90 mmHg). Patients on combination therapy were titrated from losartan 50 mg/hydrochlorothiazide 12.5 mg to losartan 50 mg/hydrochlorothiazide 12.5 mg (sham titration to maintain the blind) to losartan 100 mg/hydrochlorothiazide 25 mg. Patients on monotherapy were titrated from losartan 50 mg to losartan 100 mg to losartan 150 mg, as needed. The primary endpoint was a comparison at 4 weeks of patients who achieved goal diastolic blood pressure (trough SiDBP <90 mmHg).
The study enrolled 585 patients, including 264 (45%) females, 124 (21%) Blacks, and 21 (4%) ≥65 years of age. The mean blood pressure at baseline for the total population was 171/113 mmHg. The mean age was 53 years. After 4 weeks of therapy, the mean SiDBP was 3.1 mmHg lower and the mean SiSBP was 5.6 mmHg lower in the group treated with HYZAAR. As a result, a greater proportion of the patients on HYZAAR reached the target diastolic blood pressure (17.6% for HYZAAR, 9.4% for losartan; p=0.006). Similar trends were seen when the patients were grouped according to gender, race or age (<, ≥ 65).
After 6 weeks of therapy, more patients who received the combination regimen reached target diastolic blood pressure than those who received the monotherapy regimen (29.8% versus 12.5%).
During the study period, there were no reported cases of syncope in either treatment group. There were 2 (0.6%) and 0 (0.0%) cases of hypotension reported in the group treated with HYZAAR and the group treated with losartan, respectively. The overall pattern of adverse events reported for patients treated with HYZAAR as initial therapy was similar to the adverse event profile for patients treated with losartan as initial therapy. For information on the specific adverse events observed during the study period, see ADVERSE REACTIONS, Severe Hypertension.
-
INDICATIONS AND USAGE
Hypertension
HYZAAR is indicated for the treatment of hypertension. This fixed dose combination is not indicated for initial therapy of hypertension, except when the hypertension is severe enough that the value of achieving prompt blood pressure control exceeds the risk of initiating combination therapy in these patients (see CLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical Effects, and DOSAGE AND ADMINISTRATION).
Hypertensive Patients with Left Ventricular Hypertrophy
HYZAAR is indicated to reduce the risk of stroke in patients with hypertension and left ventricular hypertrophy, but there is evidence that this benefit does not apply to Black patients. (See PRECAUTIONS, Race, CLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical Effects, Losartan Potassium, Reduction in the Risk of Stroke,Race, and DOSAGE AND ADMINISTRATION.)
- CONTRAINDICATIONS
-
WARNINGS
Fetal/Neonatal Morbidity and Mortality
Drugs that act directly on the renin-angiotensin system can cause fetal and neonatal morbidity and death when administered to pregnant women. Several dozen cases have been reported in the world literature in patients who were taking angiotensin converting enzyme inhibitors. When pregnancy is detected, HYZAAR should be discontinued as soon as possible.
The use of drugs that act directly on the renin-angiotensin system during the second and third trimesters of pregnancy has been associated with fetal and neonatal injury, including hypotension, neonatal skull hypoplasia, anuria, reversible or irreversible renal failure, and death. Oligohydramnios has also been reported, presumably resulting from decreased fetal renal function; oligohydramnios in this setting has been associated with fetal limb contractures, craniofacial deformation, and hypoplastic lung development. Prematurity, intrauterine growth retardation, and patent ductus arteriosus have also been reported, although it is not clear whether these occurrences were due to exposure to the drug.
These adverse effects do not appear to have resulted from intrauterine drug exposure that has been limited to the first trimester.
Mothers whose embryos and fetuses are exposed to an angiotensin II receptor antagonist only during the first trimester should be so informed. Nonetheless, when patients become pregnant, physicians should have the patient discontinue the use of HYZAAR as soon as possible.
Rarely (probably less often than once in every thousand pregnancies), no alternative to an angiotensin II receptor antagonist will be found. In these rare cases, the mothers should be apprised of the potential hazards to their fetuses, and serial ultrasound examinations should be performed to assess the intra-amniotic environment.
If oligohydramnios is observed, HYZAAR should be discontinued unless it is considered life-saving for the mother. Contraction stress testing (CST), a non-stress test (NST), or biophysical profiling (BPP) may be appropriate, depending upon the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury.
Infants with histories of in utero exposure to an angiotensin II receptor antagonist should be closely observed for hypotension, oliguria, and hyperkalemia. If oliguria occurs, attention should be directed toward support of blood pressure and renal perfusion. Exchange transfusion or dialysis may be required as means of reversing hypotension and/or substituting for disordered renal function.
There was no evidence of teratogenicity in rats or rabbits treated with a maximum losartan potassium dose of 10 mg/kg/day in combination with 2.5 mg/kg/day of hydrochlorothiazide. At these dosages, respective exposures (AUCs) of losartan, its active metabolite, and hydrochlorothiazide in rabbits were approximately 5, 1.5, and 1.0 times those achieved in humans with 100 mg losartan in combination with 25 mg hydrochlorothiazide. AUC values for losartan, its active metabolite and hydrochlorothiazide, extrapolated from data obtained with losartan administered to rats at a dose of 50 mg/kg/day in combination with 12.5 mg/kg/day of hydrochlorothiazide, were approximately 6, 2, and 2 times greater than those achieved in humans with 100 mg of losartan in combination with 25 mg of hydrochlorothiazide. Fetal toxicity in rats, as evidenced by a slight increase in supernumerary ribs, was observed when females were treated prior to and throughout gestation with 10 mg/kg/day losartan in combination with 2.5 mg/kg/day hydrochlorothiazide. As also observed in studies with losartan alone, adverse fetal and neonatal effects, including decreased body weight, renal toxicity, and mortality, occurred when pregnant rats were treated during late gestation and/or lactation with 50 mg/kg/day losartan in combination with 12.5 mg/kg/day hydrochlorothiazide. Respective AUCs for losartan, its active metabolite and hydrochlorothiazide at these dosages in rats were approximately 35, 10 and 10 times greater than those achieved in humans with the administration of 100 mg of losartan in combination with 25 mg hydrochlorothiazide. When hydrochlorothiazide was administered without losartan to pregnant mice and rats during their respective periods of major organogenesis, at doses up to 3000 and 1000 mg/kg/day, respectively, there was no evidence of harm to the fetus.
Thiazides cross the placental barrier and appear in cord blood. There is a risk of fetal or neonatal jaundice, thrombocytopenia, and possibly other adverse reactions that have occurred in adults.
Hypotension — Volume-Depleted Patients
In patients who are intravascularly volume-depleted (e.g., those treated with diuretics), symptomatic hypotension may occur after initiation of therapy with HYZAAR. This condition should be corrected prior to administration of HYZAAR (see DOSAGE AND ADMINISTRATION).
Impaired Hepatic Function
Hypersensitivity Reaction
Hypersensitivity reactions to hydrochlorothiazide may occur in patients with or without a history of allergy or bronchial asthma, but are more likely in patients with such a history.
Systemic Lupus Erythematosus
Thiazide diuretics have been reported to cause exacerbation or activation of systemic lupus erythematosus.
Lithium Interaction
Lithium generally should not be given with thiazides (see PRECAUTIONS, Drug Interactions, Hydrochlorothiazide, Lithium).
-
PRECAUTIONS
General
Hypersensitivity: Angioedema. See ADVERSE REACTIONS, Post-Marketing Experience.
Losartan Potassium-Hydrochlorothiazide
In double-blind clinical trials of various doses of losartan potassium and hydrochlorothiazide, the incidence of hypertensive patients who developed hypokalemia (serum potassium <3.5 mEq/L) was 6.7% versus 3.5% for placebo; the incidence of hyperkalemia (serum potassium >5.7 mEq/L) was 0.4%. No patient discontinued due to increases or decreases in serum potassium. The mean decrease in serum potassium in patients treated with various doses of losartan and hydrochlorothiazide was 0.123 mEq/L. In patients treated with various doses of losartan and hydrochlorothiazide, there was also a dose-related decrease in the hypokalemic response to hydrochlorothiazide as the dose of losartan was increased, as well as a dose-related decrease in serum uric acid with increasing doses of losartan.
Hydrochlorothiazide
Periodic determination of serum electrolytes to detect possible electrolyte imbalance should be performed at appropriate intervals.
All patients receiving thiazide therapy should be observed for clinical signs of fluid or electrolyte imbalance: hyponatremia, hypochloremic alkalosis, and hypokalemia. Serum and urine electrolyte determinations are particularly important when the patient is vomiting excessively or receiving parenteral fluids. Warning signs or symptoms of fluid and electrolyte imbalance, irrespective of cause, include dryness of mouth, thirst, weakness, lethargy, drowsiness, restlessness, confusion, seizures, muscle pains or cramps, muscular fatigue, hypotension, oliguria, tachycardia, and gastrointestinal disturbances such as nausea and vomiting.
Hypokalemia may develop, especially with brisk diuresis, when severe cirrhosis is present, or after prolonged therapy.
Interference with adequate oral electrolyte intake will also contribute to hypokalemia. Hypokalemia may cause cardiac arrhythmia and may also sensitize or exaggerate the response of the heart to the toxic effects of digitalis (e.g., increased ventricular irritability).
Although any chloride deficit is generally mild and usually does not require specific treatment except under extraordinary circumstances (as in liver disease or renal disease), chloride replacement may be required in the treatment of metabolic alkalosis.
Dilutional hyponatremia may occur in edematous patients in hot weather; appropriate therapy is water restriction, rather than administration of salt except in rare instances when the hyponatremia is life-threatening. In actual salt depletion, appropriate replacement is the therapy of choice.
Hyperuricemia may occur or frank gout may be precipitated in certain patients receiving thiazide therapy. Because losartan decreases uric acid, losartan in combination with hydrochlorothiazide attenuates the diuretic-induced hyperuricemia.
In diabetic patients, dosage adjustments of insulin or oral hypoglycemic agents may be required. Hyperglycemia may occur with thiazide diuretics. Thus latent diabetes mellitus may become manifest during thiazide therapy.
The antihypertensive effects of the drug may be enhanced in the postsympathectomy patient.
If progressive renal impairment becomes evident, consider withholding or discontinuing diuretic therapy.
Thiazides have been shown to increase the urinary excretion of magnesium; this may result in hypomagnesemia.
Thiazides may decrease urinary calcium excretion. Thiazides may cause intermittent and slight elevation of serum calcium in the absence of known disorders of calcium metabolism. Marked hypercalcemia may be evidence of hidden hyperparathyroidism. Thiazides should be discontinued before carrying out tests for parathyroid function.
Increases in cholesterol and triglyceride levels may be associated with thiazide diuretic therapy.
Impaired Renal Function
As a consequence of inhibiting the renin-angiotensin-aldosterone system, changes in renal function have been reported in susceptible individuals treated with losartan; in some patients, these changes in renal function were reversible upon discontinuation of therapy.
In patients whose renal function may depend on the activity of the renin-angiotensin-aldosterone system (e.g., patients with severe congestive heart failure), treatment with angiotensin converting enzyme inhibitors has been associated with oliguria and/or progressive azotemia and (rarely) with acute renal failure and/or death. Similar outcomes have been reported with losartan.
In studies of ACE inhibitors in patients with unilateral or bilateral renal artery stenosis, increases in serum creatinine or BUN have been reported. Similar effects have been reported with losartan; in some patients, these effects were reversible upon discontinuation of therapy.
Thiazides should be used with caution in severe renal disease. In patients with renal disease, thiazides may precipitate azotemia. Cumulative effects of the drug may develop in patients with impaired renal function.
Information for Patients
Pregnancy: Female patients of childbearing age should be told about the consequences of second- and third-trimester exposure to drugs that act on the renin-angiotensin system, and they should also be told that these consequences do not appear to have resulted from intrauterine drug exposure that has been limited to the first trimester. These patients should be asked to report pregnancies to their physicians as soon as possible.
Symptomatic Hypotension: A patient receiving HYZAAR should be cautioned that lightheadedness can occur, especially during the first days of therapy, and that it should be reported to the prescribing physician. The patients should be told that if syncope occurs, HYZAAR should be discontinued until the physician has been consulted.
All patients should be cautioned that inadequate fluid intake, excessive perspiration, diarrhea, or vomiting can lead to an excessive fall in blood pressure, with the same consequences of lightheadedness and possible syncope.
Potassium Supplements: A patient receiving HYZAAR should be told not to use potassium supplements or salt substitutes containing potassium without consulting the prescribing physician (see PRECAUTIONS, Drug Interactions, Losartan Potassium).
Drug Interactions
Losartan Potassium
No significant drug-drug pharmacokinetic interactions have been found in interaction studies with hydrochlorothiazide, digoxin, warfarin, cimetidine and phenobarbital. Rifampin, an inducer of drug metabolism, decreased the concentrations of losartan and its active metabolite. (See CLINICAL PHARMACOLOGY, Drug Interactions.) In humans, two inhibitors of P450 3A4 have been studied. Ketoconazole did not affect the conversion of losartan to the active metabolite after intravenous administration of losartan, and erythromycin had no clinically significant effect after oral administration. Fluconazole, an inhibitor of P450 2C9, decreased active metabolite concentration and increased losartan concentration. The pharmacodynamic consequences of concomitant use of losartan and inhibitors of P450 2C9 have not been examined. Subjects who do not metabolize losartan to active metabolite have been shown to have a specific, rare defect in cytochrome P450 2C9. These data suggest that the conversion of losartan to its active metabolite is mediated primarily by P450 2C9 and not P450 3A4.
As with other drugs that block angiotensin II or its effects, concomitant use of potassium-sparing diuretics (e.g., spironolactone, triamterene, amiloride), potassium supplements, or salt substitutes containing potassium may lead to increases in serum potassium (see PRECAUTIONS, Information for Patients, Potassium Supplements).
Lithium: As with other drugs which affect the excretion of sodium, lithium excretion may be reduced. Therefore, serum lithium levels should be monitored carefully if lithium salts are to be co-administered with angiotensin II receptor antagonists.
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors): In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, co-administration of NSAIDs, including selective COX-2 inhibitors, with angiotensin II receptor antagonists (including losartan) may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving losartan and NSAID therapy.
The antihypertensive effect of angiotensin II receptor antagonists, including losartan, may be attenuated by NSAIDs, including selective COX-2 inhibitors.
Hydrochlorothiazide
When administered concurrently, the following drugs may interact with thiazide diuretics:
Alcohol, barbiturates, or narcotics — potentiation of orthostatic hypotension may occur.
Antidiabetic drugs (oral agents and insulin) — dosage adjustment of the antidiabetic drug may be required.
Other antihypertensive drugs — additive effect or potentiation.
Cholestyramine and colestipol resins — Absorption of hydrochlorothiazide is impaired in the presence of anionic exchange resins. Single doses of either cholestyramine or colestipol resins bind the hydrochlorothiazide and reduce its absorption from the gastrointestinal tract by up to 85 and 43 percent, respectively.
Corticosteroids, ACTH — intensified electrolyte depletion, particularly hypokalemia.
Pressor amines (e.g., norepinephrine) — possible decreased response to pressor amines but not sufficient to preclude their use.
Skeletal muscle relaxants, nondepolarizing (e.g., tubocurarine) — possible increased responsiveness to the muscle relaxant.
Lithium — should not generally be given with diuretics. Diuretic agents reduce the renal clearance of lithium and add a high risk of lithium toxicity. Refer to the package insert for lithium preparations before use of such preparations with HYZAAR.
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors) — The administration of a non-steroidal anti-inflammatory agent, including a selective cyclooxygenase-2 inhibitor, can reduce the diuretic, natriuretic, and antihypertensive effects of loop, potassium-sparing and thiazide diuretics. Therefore, when HYZAAR and non-steroidal anti-inflammatory agents, including selective cyclooxygenase-2 inhibitors, are used concomitantly, the patient should be observed closely to determine if the desired effect of the diuretic is obtained.
In patients receiving diuretic therapy, co-administration of NSAIDs with angiotensin receptor blockers, including losartan, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving hydrochlorothiazide, losartan, and NSAID therapy.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Losartan Potassium-Hydrochlorothiazide
No carcinogenicity studies have been conducted with the losartan potassium-hydrochlorothiazide combination.
Losartan potassium-hydrochlorothiazide when tested at a weight ratio of 4:1 was negative in the Ames microbial mutagenesis assay and the V-79 Chinese hamster lung cell mutagenesis assay. In addition, there was no evidence of direct genotoxicity in the in vitro alkaline elution assay in rat hepatocytes and in vitro chromosomal aberration assay in Chinese hamster ovary cells at noncytotoxic concentrations.
Losartan potassium, coadministered with hydrochlorothiazide, had no effect on the fertility or mating behavior of male rats at dosages up to 135 mg/kg/day of losartan and 33.75 mg/kg/day of hydrochlorothiazide. These dosages have been shown to provide respective systemic exposures (AUCs) for losartan, its active metabolite and hydrochlorothiazide that are approximately 60, 60 and 30 times greater than those achieved in humans with 100 mg of losartan potassium in combination with 25 mg of hydrochlorothiazide. In female rats, however, the coadministration of doses as low as 10 mg/kg/day of losartan and 2.5 mg/kg/day of hydrochlorothiazide was associated with slight but statistically significant decreases in fecundity and fertility indices. AUC values for losartan, its active metabolite and hydrochlorothiazide, extrapolated from data obtained with losartan administered to rats at a dose of 50 mg/kg/day in combination with 12.5 mg/kg/day of hydrochlorothiazide, were approximately 6, 2, and 2 times greater than those achieved in humans with 100 mg of losartan in combination with 25 mg of hydrochlorothiazide.
Losartan Potassium
Losartan potassium was not carcinogenic when administered at maximally tolerated dosages to rats and mice for 105 and 92 weeks, respectively. Female rats given the highest dose (270 mg/kg/day) had a slightly higher incidence of pancreatic acinar adenoma. The maximally tolerated dosages (270 mg/kg/day in rats, 200 mg/kg/day in mice) provided systemic exposures for losartan and its pharmacologically active metabolite that were approximately 160 and 90 times (rats) and 30 and 15 times (mice) the exposure of a 50 kg human given 100 mg per day.
Losartan potassium was negative in the microbial mutagenesis and V-79 mammalian cell mutagenesis assays and in the in vitro alkaline elution and in vitro and in vivo chromosomal aberration assays. In addition, the active metabolite showed no evidence of genotoxicity in the microbial mutagenesis, in vitro alkaline elution, and in vitro chromosomal aberration assays.
Fertility and reproductive performance were not affected in studies with male rats given oral doses of losartan potassium up to approximately 150 mg/kg/day. The administration of toxic dosage levels in females (300/200 mg/kg/day) was associated with a significant (p<0.05) decrease in the number of corpora lutea/female, implants/female, and live fetuses/female at C-section. At 100 mg/kg/day only a decrease in the number of corpora lutea/female was observed. The relationship of these findings to drug-treatment is uncertain since there was no effect at these dosage levels on implants/pregnant female, percent post-implantation loss, or live animals/litter at parturition. In nonpregnant rats dosed at 135 mg/kg/day for 7 days, systemic exposure (AUCs) for losartan and its active metabolite were approximately 66 and 26 times the exposure achieved in man at the maximum recommended human daily dosage (100 mg).
Hydrochlorothiazide
Two-year feeding studies in mice and rats conducted under the auspices of the National Toxicology Program (NTP) uncovered no evidence of a carcinogenic potential of hydrochlorothiazide in female mice (at doses of up to approximately 600 mg/kg/day) or in male and female rats (at doses of up to approximately 100 mg/kg/day). The NTP, however, found equivocal evidence for hepatocarcinogenicity in male mice.
Hydrochlorothiazide was not genotoxic in vitro in the Ames mutagenicity assay of Salmonella typhimurium strains TA 98, TA 100, TA 1535, TA 1537, and TA 1538 and in the Chinese Hamster Ovary (CHO) test for chromosomal aberrations, or in vivo in assays using mouse germinal cell chromosomes, Chinese hamster bone marrow chromosomes, and the Drosophila sex-linked recessive lethal trait gene. Positive test results were obtained only in the in vitro CHO Sister Chromatid Exchange (clastogenicity) and in the Mouse Lymphoma Cell (mutagenicity) assays, using concentrations of hydrochlorothiazide from 43 to 1300 μg/mL, and in the Aspergillus nidulans non-disjunction assay at an unspecified concentration.
Hydrochlorothiazide had no adverse effects on the fertility of mice and rats of either sex in studies wherein these species were exposed, via their diet, to doses of up to 100 and 4 mg/kg, respectively, prior to mating and throughout gestation.
Pregnancy
Pregnancy Categories C (first trimester) and D (second and third trimesters). See WARNINGS, Fetal/Neonatal Morbidity and Mortality.
Nursing Mothers
It is not known whether losartan is excreted in human milk, but significant levels of losartan and its active metabolite were shown to be present in rat milk. Thiazides appear in human milk. Because of the potential for adverse effects on the nursing infant, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Geriatric Use
In a controlled clinical study for the reduction in the combined risk of cardiovascular death, stroke and myocardial infarction in hypertensive patients with left ventricular hypertrophy, 2857 patients (62%) were 65 years and over, while 808 patients (18%) were 75 years and over. In an effort to control blood pressure in this study, patients were coadministered losartan and hydrochlorothiazide 74% of the total time they were on study drug. No overall differences in effectiveness were observed between these patients and younger patients. Adverse events were somewhat more frequent in the elderly compared to non-elderly patients for both the losartan-hydrochlorothiazide and the control groups (see CLINICAL PHARMACOLOGY, Special Populations).
Race
In the LIFE study, Black patients with hypertension and left ventricular hypertrophy had a lower risk of stroke on atenolol than on losartan (both cotreated with hydrochlorothiazide in the majority of patients). Given the difficulty in interpreting subset differences in large trials, it cannot be known whether the observed difference is the result of chance. However, the LIFE study does not provide evidence that the benefits of losartan on reducing the risk of cardiovascular events in hypertensive patients with left ventricular hypertrophy apply to Black patients. (See CLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical Effects; Losartan Potassium, Reduction in the Risk of Stroke.)
-
ADVERSE REACTIONS
Losartan potassium-hydrochlorothiazide has been evaluated for safety in 858 patients treated for essential hypertension and 3889 patients treated for hypertension and left ventricular hypertrophy. In clinical trials with losartan potassium-hydrochlorothiazide, no adverse experiences peculiar to this combination have been observed. Adverse experiences have been limited to those that were reported previously with losartan potassium and/or hydrochlorothiazide. The overall incidence of adverse experiences reported with the combination was comparable to placebo.
In general, treatment with losartan potassium-hydrochlorothiazide was well tolerated. For the most part, adverse experiences have been mild and transient in nature and have not required discontinuation of therapy. In controlled clinical trials, discontinuation of therapy due to clinical adverse experiences was required in only 2.8% and 2.3% of patients treated with the combination and placebo, respectively.
In these double-blind controlled clinical trials, the following adverse experiences reported with losartan-hydrochlorothiazide occurred in ≥1 percent of patients, and more often on drug than placebo, regardless of drug relationship:
Losartan Potassium-
Hydrochlorothiazide
(n=858)Placebo
(n=173)Body as a Whole
Abdominal pain
Edema/swelling
1.2
1.3
0.6
1.2Cardiovascular
Palpitation
1.4
0.0Musculoskeletal
Back pain
2.1
0.6Nervous/Psychiatric
Dizziness
5.7
2.9Respiratory
Cough
Sinusitis
Upper respiratory infection
2.6
1.2
6.1
2.3
0.6
4.6Skin
Rash
1.4
0.0The following adverse events were also reported at a rate of 1% or greater, but were as, or more, common in the placebo group in studies of essential hypertension: asthenia/fatigue, diarrhea, nausea, headache, bronchitis, pharyngitis.
Adverse events occurred at about the same rates in men and women. Adverse events were somewhat more frequent in the elderly compared to non-elderly patients and somewhat more frequent in Blacks compared to non-Blacks for both the losartan-hydrochlorothiazide and the control groups.
A patient with known hypersensitivity to aspirin and penicillin, when treated with losartan potassium, was withdrawn from study due to swelling of the lips and eyelids and facial rash, reported as angioedema, which returned to normal 5 days after therapy was discontinued.
Superficial peeling of palms and hemolysis were reported in one subject treated with losartan potassium.
Losartan Potassium
Other adverse experiences that have been reported with losartan, without regard to causality, are listed below:
Body as a Whole: chest pain, facial edema, fever, orthostatic effects, syncope; Cardiovascular: angina pectoris, arrhythmias including atrial fibrillation, sinus bradycardia, tachycardia, ventricular tachycardia and ventricular fibrillation, CVA, hypotension, myocardial infarction, second degree AV block; Digestive: anorexia, constipation, dental pain, dry mouth, dyspepsia, flatulence, gastritis, vomiting; General disorders and administration site conditions: malaise; Hematologic: anemia; Metabolic: gout; Musculoskeletal: arm pain, arthralgia, arthritis, fibromyalgia, hip pain, joint swelling, knee pain, leg pain, muscle cramps, muscle weakness, musculoskeletal pain, myalgia, shoulder pain, stiffness; Nervous System/Psychiatric: anxiety, anxiety disorder, ataxia, confusion, depression, dream abnormality, hypesthesia, insomnia, libido decreased, memory impairment, migraine, nervousness, panic disorder, paresthesia, peripheral neuropathy, sleep disorder, somnolence, tremor, vertigo; Respiratory: dyspnea, epistaxis, nasal congestion, pharyngeal discomfort, respiratory congestion, rhinitis, sinus disorder; Skin: alopecia, dermatitis, dry skin, ecchymosis, erythema, flushing, photosensitivity, pruritus, sweating, urticaria; Special Senses: blurred vision, burning/stinging in the eye, conjunctivitis, decrease in visual acuity, taste perversion, tinnitus; Urogenital: impotence, nocturia, urinary frequency, urinary tract infection.
Hydrochlorothiazide
Other adverse experiences that have been reported with hydrochlorothiazide, without regard to causality, are listed below:
Body as a Whole: weakness; Digestive: pancreatitis, jaundice (intrahepatic cholestatic jaundice), sialadenitis, cramping, gastric irritation; Hematologic: aplastic anemia, agranulocytosis, leukopenia, hemolytic anemia, thrombocytopenia; Hypersensitivity: purpura, photosensitivity, urticaria, necrotizing angiitis (vasculitis and cutaneous vasculitis), fever, respiratory distress including pneumonitis and pulmonary edema; Metabolic: hyperglycemia, glycosuria, hyperuricemia; Musculoskeletal: muscle spasm; Nervous System/Psychiatric: restlessness; Renal: renal failure, renal dysfunction, interstitial nephritis; Skin: erythema multiforme including Stevens-Johnson syndrome, exfoliative dermatitis including toxic epidermal necrolysis; Special Senses: transient blurred vision, xanthopsia.
Persistent dry cough (with an incidence of a few percent) has been associated with ACE-inhibitor use and in practice can be a cause of discontinuation of ACE-inhibitor therapy. Two prospective, parallel-group, double-blind, randomized, controlled trials were conducted to assess the effects of losartan on the incidence of cough in hypertensive patients who had experienced cough while receiving ACE-inhibitor therapy. Patients who had typical ACE-inhibitor cough when challenged with lisinopril, whose cough disappeared on placebo, were randomized to losartan 50 mg, lisinopril 20 mg, or either placebo (one study, n=97) or 25 mg hydrochlorothiazide (n=135). The double-blind treatment period lasted up to 8 weeks. The incidence of cough is shown below.
- * Demographics = (89% Caucasian, 64% female)
- † Demographics = (90% Caucasian, 51% female)
Study 1* HCTZ Losartan Lisinopril Cough 25% 17% 69% Study 2† Placebo Losartan Lisinopril Cough 35% 29% 62% These studies demonstrate that the incidence of cough associated with losartan therapy, in a population that all had cough associated with ACE-inhibitor therapy, is similar to that associated with hydrochlorothiazide or placebo therapy.
Cases of cough, including positive re-challenges, have been reported with the use of losartan in post-marketing experience.
Severe Hypertension
In a clinical study in patients with severe hypertension (SiDBP ≥110 mmHg), the overall pattern of adverse events reported through six weeks of follow-up was similar in patients treated with HYZAAR as initial therapy and in patients treated with losartan as initial therapy. There were no reported cases of syncope in either treatment group. There were 2 (0.6%) and 0 (0.0%) cases of hypotension reported in the group treated with HYZAAR and the group treated with losartan, respectively. There were 3 (0.8%) and 2 (1.2%) cases of increased serum creatinine (>0.5 mg/dL) in the group treated with HYZAAR and the group treated with losartan, respectively, during the same time period. (See CLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical Effects, Severe Hypertension.)
Post-Marketing Experience
The following additional adverse reactions have been reported in post-marketing experience:
Digestive: Hepatitis has been reported rarely in patients treated with losartan.
Hemic: Thrombocytopenia.
Hypersensitivity: Angioedema, including swelling of the larynx and glottis, causing airway obstruction and/or swelling of the face, lips, pharynx, and/or tongue has been reported rarely in patients treated with losartan; some of these patients previously experienced angioedema with other drugs including ACE inhibitors. Vasculitis, including Henoch-Schönlein purpura, has been reported with losartan. Anaphylactic reactions have been reported.
Metabolic and Nutrition: Hyperkalemia, hyponatremia have been reported with losartan.
Musculoskeletal: Rare cases of rhabdomyolysis have been reported in patients receiving angiotensin II receptor blockers.
Respiratory: Dry cough (see above) has been reported with losartan.
Skin: Erythroderma has been reported with losartan.
Laboratory Test Findings
In controlled clinical trials, clinically important changes in standard laboratory parameters were rarely associated with administration of HYZAAR.
Creatinine, Blood Urea Nitrogen: Minor increases in blood urea nitrogen (BUN) or serum creatinine were observed in 0.6 and 0.8 percent, respectively, of patients with essential hypertension treated with HYZAAR alone. No patient discontinued taking HYZAAR due to increased BUN. One patient discontinued taking HYZAAR due to a minor increase in serum creatinine.
Hemoglobin and Hematocrit: Small decreases in hemoglobin and hematocrit (mean decreases of approximately 0.14 grams percent and 0.72 volume percent, respectively) occurred frequently in patients treated with HYZAAR alone, but were rarely of clinical importance. No patients were discontinued due to anemia.
Liver Function Tests: Occasional elevations of liver enzymes and/or serum bilirubin have occurred. In patients with essential hypertension treated with HYZAAR alone, no patients were discontinued due to these laboratory adverse experiences.
Serum Electrolytes: See PRECAUTIONS.
-
OVERDOSAGE
Losartan Potassium
Significant lethality was observed in mice and rats after oral administration of 1000 mg/kg and 2000 mg/kg, respectively, about 44 and 170 times the maximum recommended human dose on a mg/m2 basis.
Limited data are available in regard to overdosage in humans. The most likely manifestation of overdosage would be hypotension and tachycardia; bradycardia could occur from parasympathetic (vagal) stimulation. If symptomatic hypotension should occur, supportive treatment should be instituted.
Neither losartan nor its active metabolite can be removed by hemodialysis.
Hydrochlorothiazide
The oral LD50 of hydrochlorothiazide is greater than 10 g/kg in both mice and rats. The most common signs and symptoms observed are those caused by electrolyte depletion (hypokalemia, hypochloremia, hyponatremia) and dehydration resulting from excessive diuresis. If digitalis has also been administered, hypokalemia may accentuate cardiac arrhythmias. The degree to which hydrochlorothiazide is removed by hemodialysis has not been established.
-
DOSAGE AND ADMINISTRATION
Hypertension
Dosing must be individualized. The usual starting dose of losartan is 50 mg once daily, with 25 mg recommended for patients with intravascular volume depletion (e.g., patients treated with diuretics) (see WARNINGS, Hypotension — Volume-Depleted Patients) and patients with a history of hepatic impairment (see WARNINGS, Impaired Hepatic Function). Losartan can be administered once or twice daily at total daily doses of 25 to 100 mg. If the antihypertensive effect measured at trough using once-a-day dosing is inadequate, a twice-a-day regimen at the same total daily dose or an increase in dose may give a more satisfactory response.
Hydrochlorothiazide is effective in doses of 12.5 to 50 mg once daily and can be given at doses of 12.5 to 25 mg as HYZAAR.
To minimize dose-independent side effects, it is usually appropriate to begin combination therapy only after a patient has failed to achieve the desired effect with monotherapy.
The side effects (see WARNINGS) of losartan are generally rare and apparently independent of dose; those of hydrochlorothiazide are a mixture of dose-dependent (primarily hypokalemia) and dose-independent phenomena (e.g., pancreatitis), the former much more common than the latter. Therapy with any combination of losartan and hydrochlorothiazide will be associated with both sets of dose-independent side effects.
Replacement Therapy: The combination may be substituted for the titrated components.
Dose Titration by Clinical Effect: A patient whose blood pressure is not adequately controlled with losartan monotherapy (see above) or hydrochlorothiazide alone may be switched to HYZAAR 50-12.5 (losartan 50 mg/hydrochlorothiazide 12.5 mg) once daily. If blood pressure remains uncontrolled after about 3 weeks of therapy, the dose may be increased to two tablets of HYZAAR 50-12.5 once daily or one tablet of HYZAAR 100-25 (losartan 100 mg/hydrochlorothiazide 25 mg) once daily. A patient whose blood pressure is not adequately controlled with losartan 100 mg monotherapy (see above) may be switched to HYZAAR 100-12.5 once daily. If blood pressure remains uncontrolled after about 3 weeks of therapy, the dose may be increased to two tablets of HYZAAR 50-12.5 once daily or one tablet of HYZAAR 100-25 (losartan 100 mg/hydrochlorothiazide 25 mg) once daily.
A patient whose blood pressure is inadequately controlled by 25 mg once daily of hydrochlorothiazide, or is controlled but who experiences hypokalemia with this regimen, may be switched to HYZAAR 50-12.5 (losartan 50 mg/hydrochlorothiazide 12.5 mg) once daily, reducing the dose of hydrochlorothiazide without reducing the overall expected antihypertensive response. The clinical response to HYZAAR 50-12.5 should be subsequently evaluated, and if blood pressure remains uncontrolled after about 3 weeks of therapy, the dose may be increased to two tablets of HYZAAR 50-12.5 once daily or one tablet of HYZAAR 100-25 (losartan 100 mg/hydrochlorothiazide 25 mg) once daily.
The usual dose of HYZAAR is one tablet of HYZAAR 50-12.5 once daily. More than two tablets of HYZAAR 50-12.5 once daily or more than one tablet of HYZAAR 100-25 once daily is not recommended. The maximal antihypertensive effect is attained about 3 weeks after initiation of therapy.
Use in Patients with Renal Impairment: The usual regimens of therapy with HYZAAR may be followed as long as the patient's creatinine clearance is >30 mL/min. In patients with more severe renal impairment, loop diuretics are preferred to thiazides, so HYZAAR is not recommended.
Patients with Hepatic Impairment: HYZAAR is not recommended for titration in patients with hepatic impairment (see WARNINGS, Impaired Hepatic Function) because the appropriate 25 mg starting dose of losartan cannot be given.
Severe Hypertension
The starting dose of HYZAAR for initial treatment of severe hypertension is one tablet of HYZAAR 50-12.5 once daily (see CLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical Effects). For patients who do not respond adequately to HYZAAR 50-12.5 after 2 to 4 weeks of therapy, the dosage may be increased to one tablet of HYZAAR 100-25 once daily. The maximum dose is one tablet of HYZAAR 100-25 once daily. HYZAAR is not recommended as initial therapy in patients with hepatic impairment (see WARNINGS, Impaired Hepatic Function) because the appropriate 25 mg starting dose of losartan cannot be given. It is also not recommended for use as initial therapy in patients with intravascular volume depletion (e.g., patients treated with diuretics, see WARNINGS, Hypotension — Volume-Depleted Patients).
Hypertensive Patients with Left Ventricular Hypertrophy
Treatment should be initiated with COZAAR 50 mg once daily. Hydrochlorothiazide 12.5 mg should be added or HYZAAR 50-12.5 substituted if the blood pressure reduction is inadequate. If additional blood pressure reduction is needed, COZAAR 100 mg and hydrochlorothiazide 12.5 mg or HYZAAR 100-12.5 may be substituted, followed by COZAAR 100 mg and hydrochlorothiazide 25 mg or HYZAAR 100-25. For further blood pressure reduction other antihypertensives should be added (see CLINICAL PHARMACOLOGY, Pharmacodynamics and Clinical Effects, Losartan Potassium, Reduction in the Risk of Stroke).
HYZAAR may be administered with other antihypertensive agents.
HYZAAR may be administered with or without food.
- HOW SUPPLIED
-
PATIENT PACKAGE INSERT
Patient Information
HYZAAR®
("HY-zar")
(losartan potassium-hydrochlorothiazide tablets)
50-12.5 mg, 100-12.5 mg, 100-25 mg
Rx onlyRead the Patient Information that comes with HYZAAR4 before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your condition and treatment.
- 4
Registered trademark of E.I. du Pont de Nemours and Company, Wilmington, Delaware, USA
Copyright © 1995, 2005 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
All rights reservedWhat is the most important information I should know about HYZAAR?
Do not take HYZAAR if you are pregnant or plan to become pregnant. HYZAAR can harm your unborn baby causing injury and even death. Stop taking HYZAAR if you become pregnant and call your doctor right away. If you plan to become pregnant, talk to your doctor about other treatment options before taking HYZAAR.
What is HYZAAR?
HYZAAR contains 2 prescription medicines, an angiotensin receptor blocker (ARB) and a diuretic (water pill). It is used to:
- lower high blood pressure (hypertension). HYZAAR is not usually the first medicine used to treat high blood pressure.
- lower the chance of stroke in patients with high blood pressure and a heart problem called left ventricular hypertrophy (LVH). HYZAAR may not help Black patients with this problem.
HYZAAR has not been studied in children less than 18 years old.
High Blood Pressure (hypertension) Blood pressure is the force in your blood vessels when your heart beats and when your heart rests. You have high blood pressure when the force is too much. The losartan ingredient in HYZAAR can help your blood vessels relax so your blood pressure is lower. The hydrochlorothiazide ingredient in HYZAAR works by making your kidneys pass more water and salt.
Left Ventricular Hypertrophy (LVH) is an enlargement of the walls of the left chamber of the heart (the heart’s main pumping chamber). LVH can happen from several things. High blood pressure is the most common cause of LVH.
Who should not take HYZAAR?
Do not take HYZAAR if you:
- are allergic to any ingredients in HYZAAR. See a complete list of ingredients in HYZAAR at the end of this leaflet.
- are allergic to any sulfonamide-containing ("sulfa") medicines. Ask your doctor if you are not sure what sulfonamide-containing ("sulfa") medicines are.
- are not passing urine.
What should I tell my doctor before taking HYZAAR?
Tell your doctor about all your medical conditions including if you:
- are pregnant or planning to become pregnant. See "What is the most important information I should know about HYZAAR?"
- are breast-feeding or plan to breast-feed. HYZAAR can pass into your milk and may harm your baby. You and your doctor should decide if you will take HYZAAR or breast-feed. You should not do both.
- have been vomiting (throwing up), having diarrhea, sweating a lot, or not drinking enough fluids. These could cause you to have low blood pressure.
- have liver problems
- have kidney problems
- have systemic lupus erythematosus (Lupus; SLE)
- have diabetes
- have asthma
- have gout
- have any allergies
Tell your doctor about all of the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
HYZAAR and certain other medicines may interact with each other. Especially tell your doctor if you are taking:
- potassium supplements
- salt substitutes containing potassium
- water pills (diuretics)
- lithium (a medicine used to treat a certain kind of depression)
- medicines used to treat pain and arthritis, called non-steroidal anti-inflammatory drugs (NSAIDs), including COX-2 inhibitors.
Know the medicines you take. Keep a list of your medicines and show it to your doctor and pharmacist when you get a new medicine.
How should I take HYZAAR?
- Take HYZAAR exactly as prescribed by your doctor. Your doctor may change your dose if needed.
- HYZAAR can be taken with or without food.
- If you miss a dose, take it as soon as you remember. If it is close to your next dose, do not take the missed dose. Just take the next dose at your regular time.
- If you take too much HYZAAR, call your doctor or Poison Control Center, or go to the nearest hospital emergency room right away.
- Your doctor may do blood tests from time to time while you are taking HYZAAR.
What are the possible side effects of HYZAAR?
HYZAAR may cause the following side effects that may be serious:
- injury or death of unborn babies. See "What is the most important information I should know about HYZAAR?"
- allergic reaction. Symptoms of an allergic reaction are swelling of the face, lips, throat, or tongue. Get emergency medical help right away and stop taking HYZAAR.
- low blood pressure (hypotension). Low blood pressure may cause you to feel faint or dizzy. Lie down if you feel faint or dizzy. Call your doctor right away.
- a new or worsening condition called systemic lupus erythematosus (Lupus; SLE)
- if you have kidney problems, you may see a worsening in how well your kidneys work. Call your doctor if you get swelling in your feet, ankles, or hands, or unexplained weight gain.
- If you have liver problems, you may see a worsening in how well your liver works. Call your doctor if you get nausea, pain in the right upper stomach area (abdomen), yellow eyes or skin (which can be itchy).
The most common side effects of HYZAAR in people with high blood pressure are:
- "colds" (upper respiratory infection)
- dizziness
- stuffy nose
- back pain
- fast or irregular heartbeat (palpitations)
- rash
Tell your doctor if you get any side effect that bothers you or that won't go away. This is not a complete list of side effects. For a complete list, ask your doctor or pharmacist.
How should I store HYZAAR?
- Store HYZAAR at room temperature at 59°F to 86°F (15°C to 30°C).
- Keep HYZAAR in a tightly closed container, and keep HYZAAR out of the light.
- Keep HYZAAR and all medicines out of the reach of children.
General information about HYZAAR
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use HYZAAR for a condition for which it was not prescribed. Do not give HYZAAR to other people, even if they have the same symptoms that you have. It may harm them.
This leaflet summarizes the most important information about HYZAAR. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information that is written for health professionals.
What are the ingredients in HYZAAR?
Active ingredients: losartan potassium, hydrochlorothiazide
Inactive ingredients:
microcrystalline cellulose, lactose hydrous, pregelatinized starch, magnesium stearate, hydroxypropyl cellulose, hypromellose, titanium dioxide. HYZAAR 50-12.5 and HYZAAR 100-25 also contain D&C yellow No. 10 aluminum lake. HYZAAR 50-12.5, HYZAAR 100-12.5, and HYZAAR 100-25 may also contain carnauba wax.
Manufactured For:
Merck Sharp & Dohme Corp., a subsidiary of
Merck & Co., Inc.
Whitehouse Station, NJ 08889, USAIssued June 2010
9964302
Repackaged by:
Rebel Distributors Corp
Thousand Oaks, CA 91320
9952700
30 | No. 6517 - 4
- PRINCIPAL DISPLAY PANEL - Bottle Label 100-25
-
INGREDIENTS AND APPEARANCE
HYZAAR
losartan potassium and hydrochlorothiazide tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 21695-788(NDC:0006-0747) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength losartan potassium (UNII: 3ST302B24A) (losartan - UNII:JMS50MPO89) losartan potassium 100 mg hydrochlorothiazide (UNII: 0J48LPH2TH) (hydrochlorothiazide - UNII:0J48LPH2TH) hydrochlorothiazide 25 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) magnesium stearate (UNII: 70097M6I30) hydroxypropyl cellulose (UNII: RFW2ET671P) HYPROMELLOSES (UNII: 3NXW29V3WO) titanium dioxide (UNII: 15FIX9V2JP) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) CARNAUBA WAX (UNII: R12CBM0EIZ) Product Characteristics Color YELLOW (light yellow) Score no score Shape OVAL (oval) Size 16mm Flavor Imprint Code 747 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 21695-788-90 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020387 04/28/1995 Labeler - Rebel Distributors Corp (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp 118802834 RELABEL, REPACK
Trademark Results [HYZAAR]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 HYZAAR 74431110 1917146 Live/Registered |
MERCK SHARP & DOHME CORP. 1993-09-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.