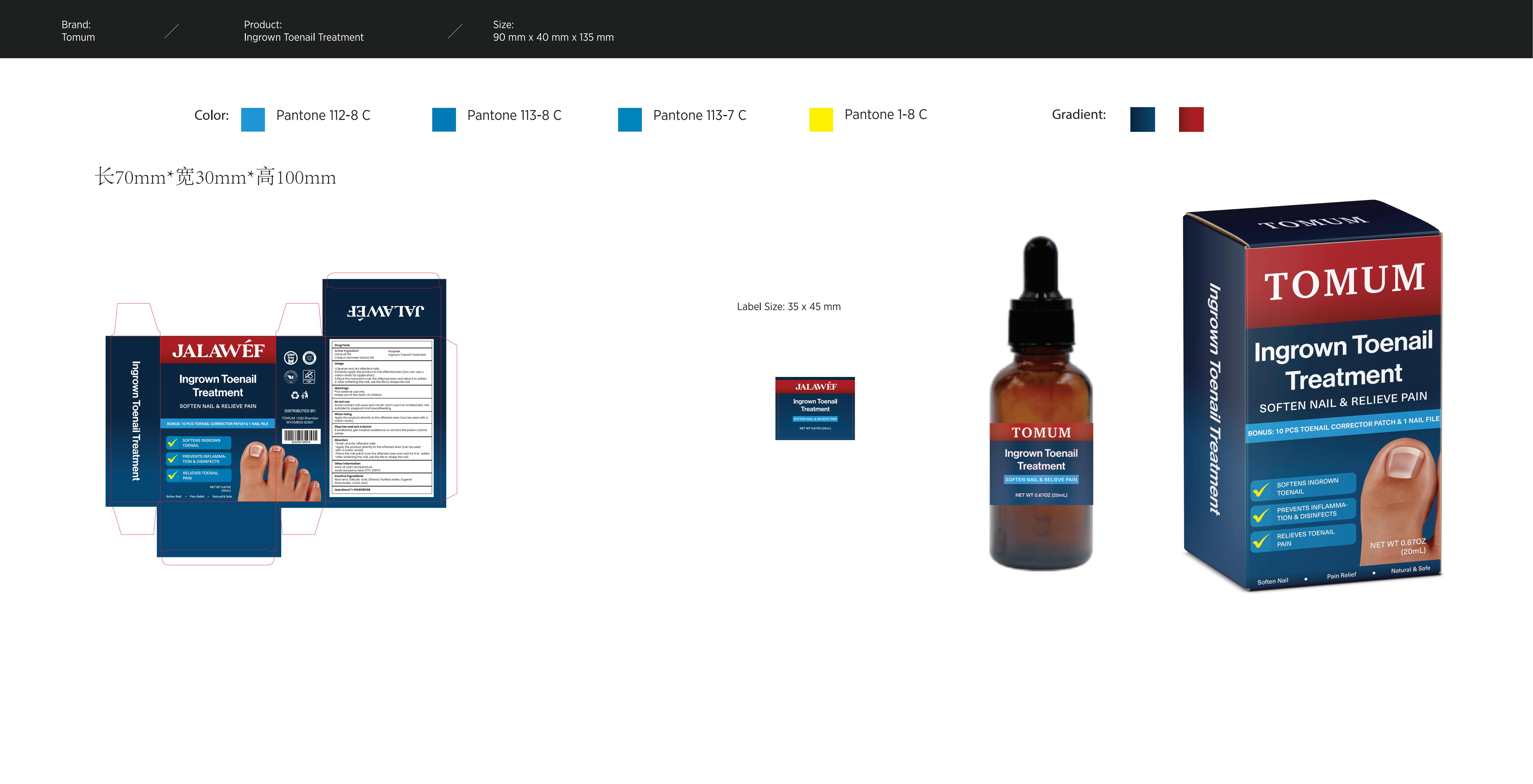
Ingrown Nail Treatment by Consilii LLC 83299-029 Completed
Ingrown Nail Treatment by
Drug Labeling and Warnings
Ingrown Nail Treatment by is a Otc medication manufactured, distributed, or labeled by Consilii LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
INGROWN NAIL TREATMENT- jalawef ingrown nail treatment liquid
Consilii LLC
----------
83299-029 Completed
Use
(Usage)
1.Cleanse and dry affected nails.
2.Directly apply the product to the affected area (you can use a cotton swab for application).
3.Place the nail patch over the affected area and allow it to soften.
4. After softening the nail, use the file to shape the nail.
Do not use
Avoidcontact with eyes and mouth. Donot use it on the irritated skin. Notsuitable for pregnant andbreastfeeding.
Directions
DIRECTION:
Wash and dry affected nails
Apply the product directly to the affected area (can be used with a cotton swab).
Place the nail patch over the affected area and wait for it to soften.
After softening the nail, use the file to shape the nail.
| INGROWN NAIL TREATMENT
jalawef ingrown nail treatment liquid |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Consilii LLC (118891890) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Consilii LLC | 118891890 | label(83299-029) , manufacture(83299-029) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.