4% Lidocaine plus 1% Menthol Pain Relief Patch by Koolcare Technology Co., Ltd.
4% Lidocaine plus 1% Menthol Pain Relief Patch by
Drug Labeling and Warnings
4% Lidocaine plus 1% Menthol Pain Relief Patch by is a Otc medication manufactured, distributed, or labeled by Koolcare Technology Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
0.025% CAPSAICIN PATCH- capsaicin pain relief patch, capsaicin pain relief strip patch
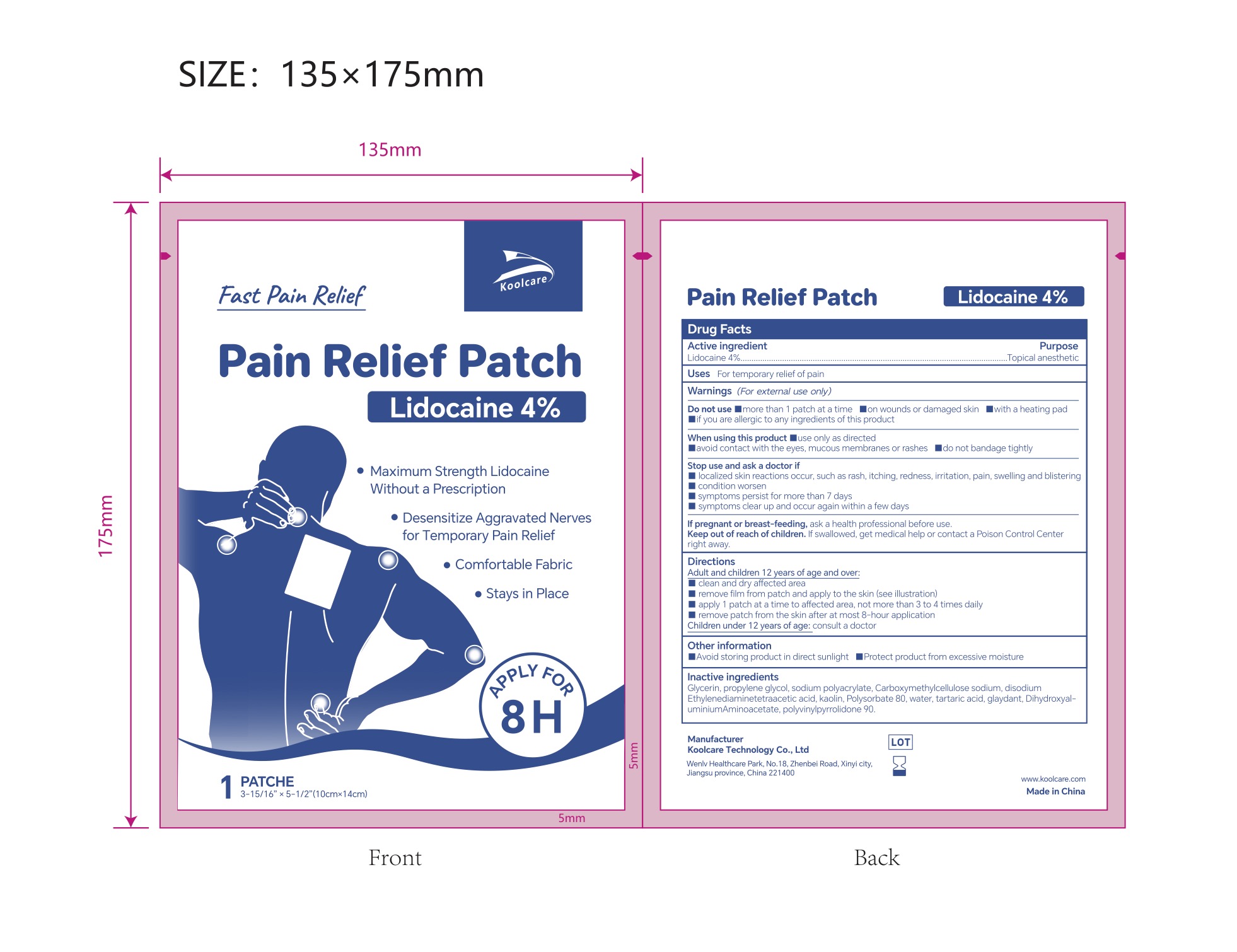
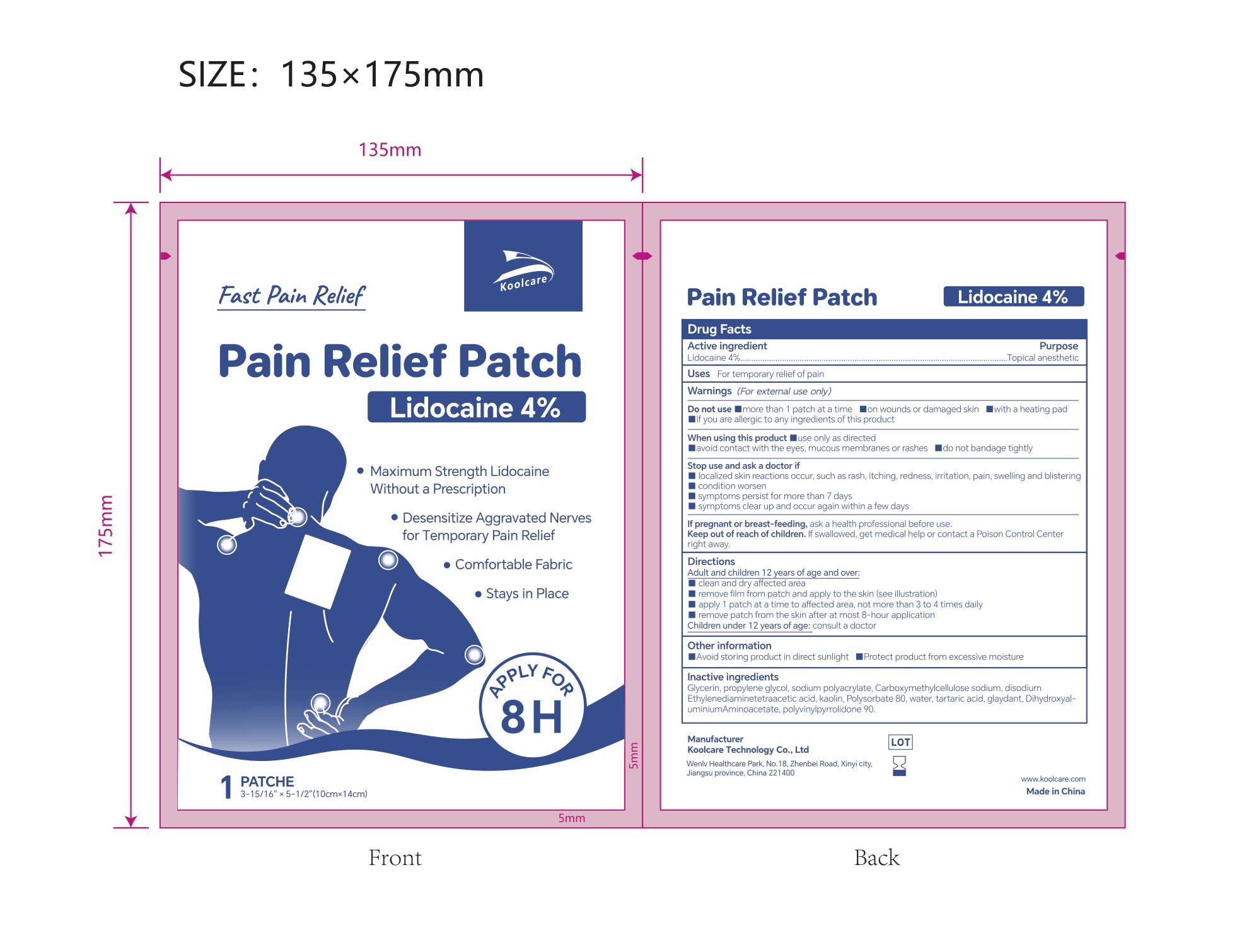
4% LIDOCAINE PAIN RELIEF PATCH- lidocaine pain relief patch patch
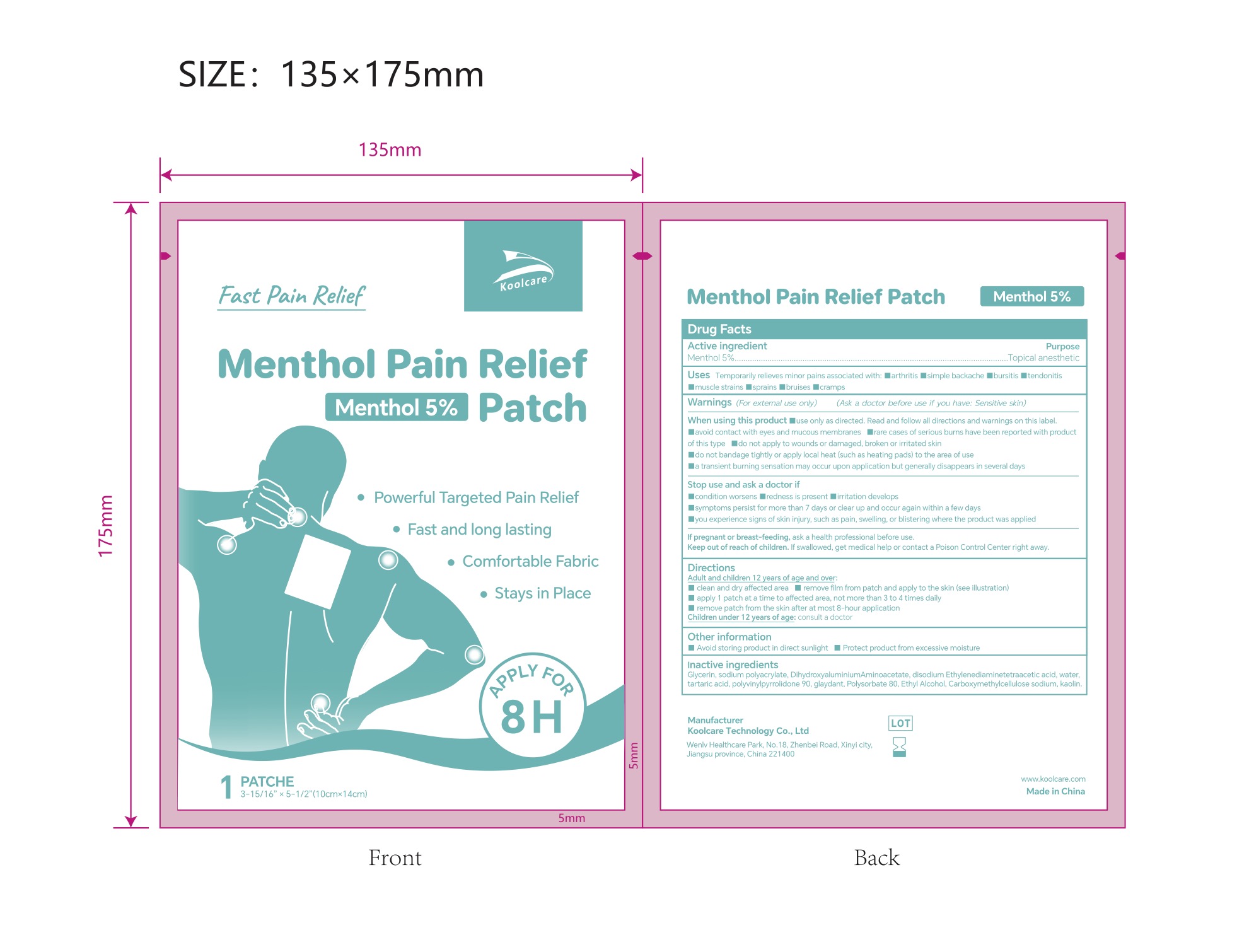
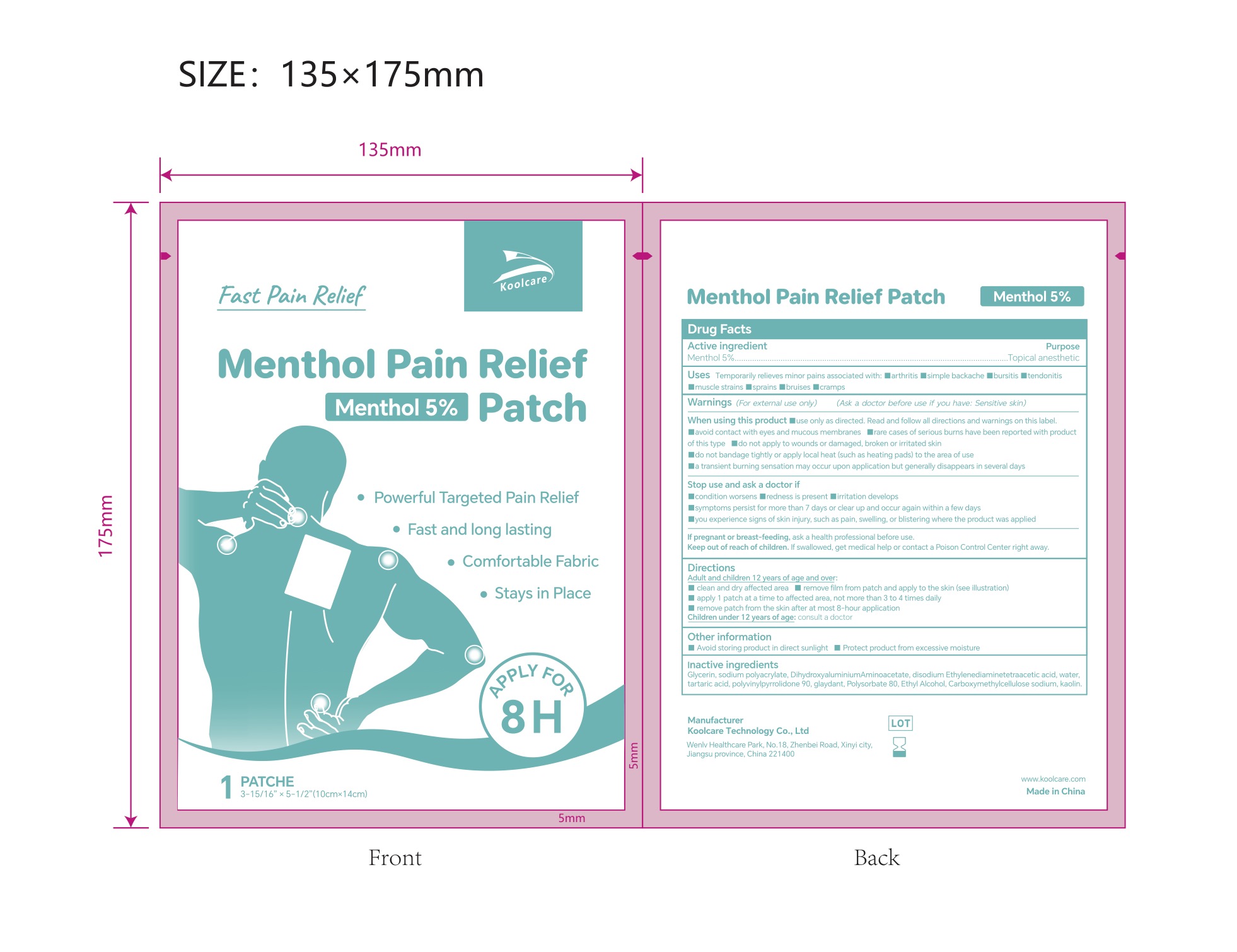
5% MENTHOL PAIN RELIEF PATCH- pain relief patch patch
4% LIDOCAINE PLUS 1% MENTHOL PAIN RELIEF PATCH- pain relief patch, pain relief strip patch
Koolcare Technology Co., Ltd.
----------
0.025% Capsaicin Patch NDC: 84205-001-00

Directions
appy 1 patch at a time to affect area, not more than 3 to 4 times daily
remove patch from the skin after a most 8-hour application

Warnings

Do not use
- more than 1 patch at a time
- on wounds or damaged skin
- with a heating pad
- if you are allergic to any ingredients of this product
When using this product
- use only as directed
- avoid contact with the eyes, mucous membranes or rashes
- do not bandage tightly
Stop use and ask a doctor if
- localized skin reactions occur, such as rash, itching, redness, irritation, pain, swelling and blistering
- condition worsen
- symptoms persist form more than 7 days
- symptoms clear up and occur again within a few days
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Posison Control Center right away.
Inactive ingredient
Glycerin, sodium polyacrylate, Carboxymethylcellulose sodium, Dihydroxyaluminium Aminoacetate, disodium Ethylenediaminetetraacetic acid, kaolin, water, tartaric acid, polyvinylpyrrolidone 90, glaydant (DMDM HYDANTOIN), propylene glycol, Polysorbate 80.
Directions
Directions
Adult and children 12 years of age and over:
- Clean and dry affected area
- Remove film from patch and apply to the skin (see illustration)
- Apply 1 patch at a time to affected area, not more than 3 to 4 times daily
- Remove patch from the skin after at most 8-hour application
Children under 12 years of age: consult a doctor
4% Lidocaine Pain Relief Patch NDC: 84205-002-00
Directions
appy 1 patch at a time to affect area, not more than 3 to 4 times daily
remove patch from the skin after a most 8-hour application
Warnings
Warnings (For external use only)
Do not use
- more than 1 patch at a time
- on wounds or damaged skin
- with a heating pad
- if you are allergic to any ingredients of this product
When using this product
- use only as directed
- avoid contact with the eyes, mucous membranes or rashes
- do not bandage tightly
Stop use and ask a doctor if
- localized skin reactions occur, such as rash, itching, redness, irritation, pain, swelling and blistering
- condition worsen
- symptoms persist form more than 7 days
- symptoms clear up and occur again within a few days
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Posison Control Center right away.
Inactive ingredients
Glycerin, propylene glycol, sodium polyacrylate, Carboxymethylcellulose sodium, disodium Ethylenediaminetetraacetic acid, kaolin, Polysorbate 80, water, tartaric acid, glaydant(DMDM HYDANTOIN), DihydroxyaluminiumAminoacetate, polyvinylpyrrolidone 90.
Directions
Directions
Adult and children 12 years of age and over:
- Clean and dry affected area
- Remove film from patch and apply to the skin (see illustration)
- Apply 1 patch at a time to affected area, not more than 3 to 4 times daily
- Remove patch from the skin after at most 8-hour application
Children under 12 years of age: consult a doctor
4% Lidocaine plus 1% Menthol Pain Relief Patch NDC: 84205-003-00

Directions
appy 1 patch at a time to affect area, not more than 3 to 4 times daily
remove patch from the skin after a most 8-hour application
Warnings
Warnings (For external use only)
Do not use
- more than 1 patch at a time
- on wounds or damaged skin
- with a heating pad
- if you are allergic to any ingredients of this product
When using this product
- use only as directed
- avoid contact with the eyes, mucous membranes or rashes
- do not bandage tightly
Stop use and ask a doctor if
- localized skin reactions occur, such as rash, itching, redness, irritation, pain, swelling and blistering
- condition worsen
- symptoms persist form more than 7 days
- symptoms clear up and occur again within a few days
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Posison Control Center right away.
Inactive ingredients
Glycerin, propylene glycol, sodium polyacrylate, Carboxymethylcellulose sodium, Dihydroxyaluminium Aminoacetate, kaolin, Ethyl Alcohol, water,tartaric acid, polyvinylpyrrolidone 90, glaydant(DMDM HYDANTOIN), disodium Ethylenediaminetetraacetic acid, Polysorbate 80.
Directions
Directions
Adult and children 12 years of age and over:
- Clean and dry affected area
- Remove film from patch and apply to the skin (see illustration)
- Apply 1 patch at a time to affected area, not more than 3 to 4 times daily
- Remove patch from the skin after at most 8-hour application
Children under 12 years of age: consult a doctor
0.025% Capsaicin Patch NDC: 84205-001-01

Inactive ingredients
Glycerin, sodium polyacrylate, Carboxymethylcellulose sodium, Dihydroxyaluminium Aminoacetate, disodium Ethylenediaminetetraacetic acid, kaolin, water, tartaric acid, polyvinylpyrrolidone 90, glaydant (DMDM HYDANTOIN), propylene glycol, Polysorbate 80.
Directions
Directions
Adult and children 12 years of age and over:
- Clean and dry affected area
- Remove film from patch and apply to the skin (see illustration)
- Apply 1 patch at a time to affected area, not more than 3 to 4 times daily
- Remove patch from the skin after at most 8-hour application
Children under 12 years of age: consult a doctor
5% Menthol Pain Relief Patch NDC: 84205-004-01

Directions
appy 1 patch at a time to affect area, not more than 3 to 4 times daily
remove patch from the skin after a most 8-hour application
Warnings
Warnings (For external use only)
Do not use
- more than 1 patch at a time
- on wounds or damaged skin
- with a heating pad
- if you are allergic to any ingredients of this product
When using this product
- use only as directed
- avoid contact with the eyes, mucous membranes or rashes
- do not bandage tightly
Stop use and ask a doctor if
- localized skin reactions occur, such as rash, itching, redness, irritation, pain, swelling and blistering
- condition worsen
- symptoms persist form more than 7 days
- symptoms clear up and occur again within a few days
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Posison Control Center right away.
Inactive ingredients
Glycerin, sodium polyacrylate, Dihydroxyaluminium Aminoa acetate, disodium Ethylenediaminetetraacetic acid, water, tartaric acid, polyvinylpyrrolidone 90, glaydant(DMDM HYDANTOIN), Polysorbate 80, Ethyl Alcohol, Carboxymethylcellulose sodium, kaolin.
Directions
Directions
Adult and children 12 years of age and over:
- Clean and dry affected area
- Remove film from patch and apply to the skin (see illustration)
- Apply 1 patch at a time to affected area, not more than 3 to 4 times daily
- Remove patch from the skin after at most 8-hour application
Children under 12 years of age: consult a doctor
5% Menthol Pain Relief Patch NDC: 84205-004-00
5% Menthol Pain Relief Patch NDC: 84205-004-00
Directions
appy 1 patch at a time to affect area, not more than 3 to 4 times daily
remove patch from the skin after a most 8-hour application
Warnings
Warnings (For external use only)
Do not use
- more than 1 patch at a time
- on wounds or damaged skin
- with a heating pad
- if you are allergic to any ingredients of this product
When using this product
- use only as directed
- avoid contact with the eyes, mucous membranes or rashes
- do not bandage tightly
Stop use and ask a doctor if
- localized skin reactions occur, such as rash, itching, redness, irritation, pain, swelling and blistering
- condition worsen
- symptoms persist form more than 7 days
- symptoms clear up and occur again within a few days
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Posison Control Center right away.
Inactive ingredients
Glycerin, sodium polyacrylate, Dihydroxyaluminium Aminoa acetate, disodium Ethylenediaminetetraacetic acid, water, tartaric acid, polyvinylpyrrolidone90, glaydant(DMDM HYDANTOIN), Polysorbate80, Ethyl Alcohol, Carboxymethylcellulose sodium, kaolin.
Directions
Directions
Adult and children 12 years of age and over:
- Clean and dry affected area
- Remove film from patch and apply to the skin (see illustration)
- Apply 1 patch at a time to affected area, not more than 3 to 4 times daily
- Remove patch from the skin after at most 8-hour application
Children under 12 years of age: consult a doctor
4% Lidocaine Pain Relief Patch NDC: 84205-002-01

Warnings
Warnings (For external use only)
Do not use
- more than 1 patch at a time
- on wounds or damaged skin
- with a heating pad
- if you are allergic to any ingredients of this product
When using this product
- use only as directed
- avoid contact with the eyes, mucous membranes or rashes
- do not bandage tightly
Stop use and ask a doctor if
- localized skin reactions occur, such as rash, itching, redness, irritation, pain, swelling and blistering
- condition worsen
- symptoms persist form more than 7 days
- symptoms clear up and occur again within a few days
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Posison Control Center right away.
Directions
appy 1 patch at a time to affect area, not more than 3 to 4 times daily
remove patch from the skin after a most 8-hour application
Inactive ingredients
Glycerin, propylene glycol, sodium polyacrylate, Carboxymethylcellulose sodium, disodium Ethylenediaminetetraacetic acid, kaolin, Polysorbate 80, water, tartaric acid, glaydant(DMDM HYDANTOIN), DihydroxyaluminiumAminoacetate, polyvinylpyrrolidone 90.
Directions
Directions
Adult and children 12 years of age and over:
- Clean and dry affected area
- Remove film from patch and apply to the skin (see illustration)
- Apply 1 patch at a time to affected area, not more than 3 to 4 times daily
- Remove patch from the skin after at most 8-hour application
Children under 12 years of age: consult a doctor
4% Lidocaine plus 1% Menthol Pain Relief Patch NDC: 84205-003-01

Directions
appy 1 patch at a time to affect area, not more than 3 to 4 times daily
remove patch from the skin after a most 8-hour application
Warnings
Warnings (For external use only)
Do not use
- more than 1 patch at a time
- on wounds or damaged skin
- with a heating pad
- if you are allergic to any ingredients of this product
When using this product
- use only as directed
- avoid contact with the eyes, mucous membranes or rashes
- do not bandage tightly
Stop use and ask a doctor if
- localized skin reactions occur, such as rash, itching, redness, irritation, pain, swelling and blistering
- condition worsen
- symptoms persist form more than 7 days
- symptoms clear up and occur again within a few days
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Posison Control Center right away.
Inactive ingredients
Glycerin, propylene glycol, sodium polyacrylate, Carboxymethylcellulose sodium, Dihydroxyaluminium Aminoacetate, kaolin, Ethyl Alcohol, water,tartaric acid, polyvinylpyrrolidone 90, glaydant(DMDM HYDANTOIN), disodium Ethylenediaminetetraacetic acid, Polysorbate 80.
Directions
Directions
Adult and children 12 years of age and over:
- Clean and dry affected area
- Remove film from patch and apply to the skin (see illustration)
- Apply 1 patch at a time to affected area, not more than 3 to 4 times daily
- Remove patch from the skin after at most 8-hour application
Children under 12 years of age: consult a doctor
| 0.025% CAPSAICIN PATCH
capsaicin pain relief patch, capsaicin pain relief strip patch |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| 4% LIDOCAINE PAIN RELIEF PATCH
lidocaine pain relief patch patch |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| 5% MENTHOL PAIN RELIEF PATCH
pain relief patch patch |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| 4% LIDOCAINE PLUS 1% MENTHOL PAIN RELIEF PATCH
pain relief patch, pain relief strip patch |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Koolcare Technology Co., Ltd. (602479389) |
| Registrant - Koolcare Technology Co., Ltd. (602479389) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Koolcare Technology Co., Ltd. | 602479389 | manufacture(84205-001, 84205-002, 84205-004, 84205-003) | |