SPIKEVAX by Catalent Indiana, LLC 256-109-301
SPIKEVAX by
Drug Labeling and Warnings
SPIKEVAX by is a Other medication manufactured, distributed, or labeled by Catalent Indiana, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
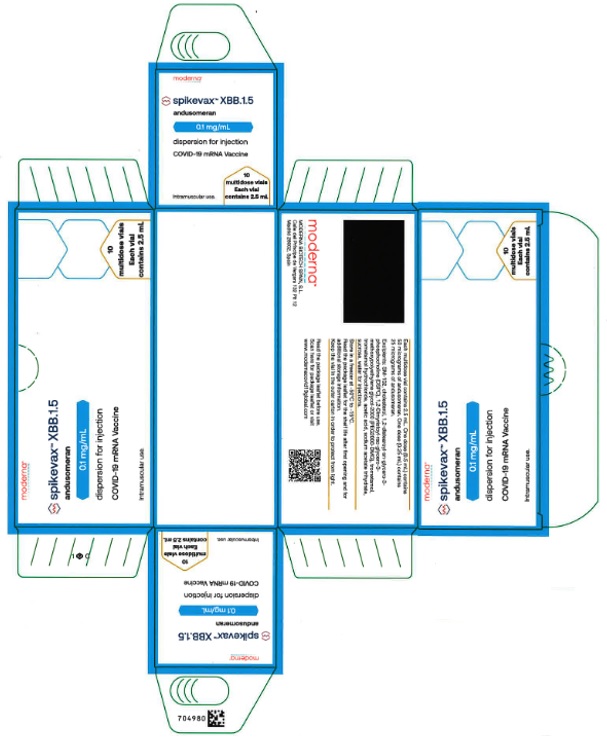
SPIKEVAX- covid-19 vaccine, mrna injection, solution
Catalent Indiana, LLC
----------
256-109-301
| SPIKEVAX
covid-19 vaccine, mrna injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Catalent Indiana, LLC (172209277) |
Revised: 8/2025
Document Id: 3c675d4f-7134-3cd7-e063-6294a90afb44
Set id: 1aea6699-253b-4007-b383-2f9a204a42e5
Version: 8
Effective Time: 20250815
Trademark Results [SPIKEVAX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SPIKEVAX 97037862 not registered Live/Pending |
ModernaTx, Inc. 2021-09-21 |
 SPIKEVAX 90247367 not registered Live/Pending |
ModernaTx, Inc. 2020-10-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.