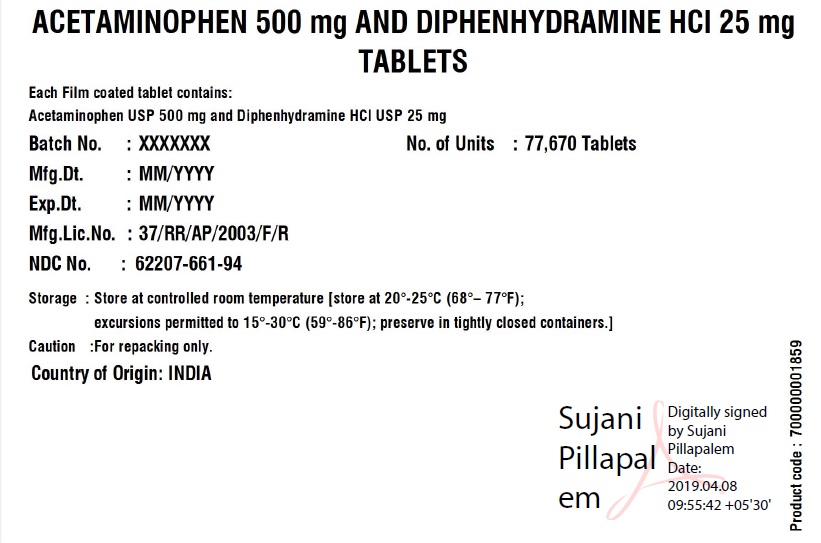
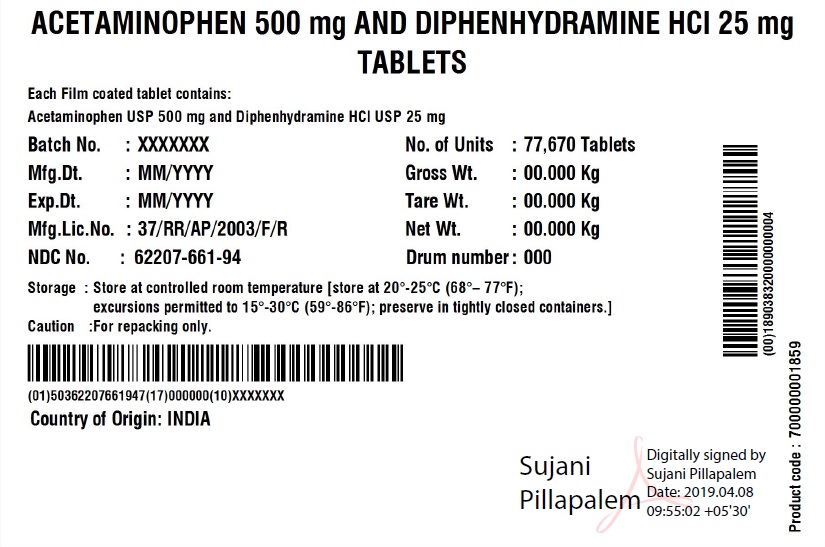
Acetaminophen 500mg Diphenhydramine HCl 25mg Tablets
Acetaminophen and Diphenhydramine Hydrochloride by
Drug Labeling and Warnings
Acetaminophen and Diphenhydramine Hydrochloride by is a Other medication manufactured, distributed, or labeled by Granules India Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ACETAMINOPHEN AND DIPHENHYDRAMINE HYDROCHLORIDE- acetaminophen and diphenhydramine hydrochloride tablet
Granules India Limited
----------
Acetaminophen 500mg Diphenhydramine HCl 25mg Tablets
USES
temporary relief of occasional headaches and minor aches and pains with accompanying sleeplessness
WARNINGS
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
DO NOT USE
- with any other drug containing acetaminophen (prescription or nonprescription).If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- with any product containing diphenhydramine, even one used on skin
- in children under 12 years of age
- if you have ever had an allergic reaction to this product or any of its ingredients
ASK A DOCTOR
before use if you have
- liver disease
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
- glaucoma
ASK A DOCTOR OR PHARMACIST
before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
WHEN USING
this product
- drowsiness will occur
- avoid alcoholic drinks
- do not drive a motor vehicle or operate machinery
STOP USE
and ask a doctor if
- sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of serious underlying medical illness.
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
These could be signs of a serious condition.
KEEP OUT OF REACH OF CHILDREN
Overdose warning Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222) Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
DIRECTIONS
Directions
- do not take more than directed (see overdose warning)
| adults and children 12 years and over |
|
| children under 12 years |
|
| ACETAMINOPHEN AND DIPHENHYDRAMINE HYDROCHLORIDE
acetaminophen and diphenhydramine hydrochloride tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Granules India Limited (915000087) |
| Registrant - Granules India Limited (915000087) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Granules India Limited | 918609236 | manufacture(62207-661) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.