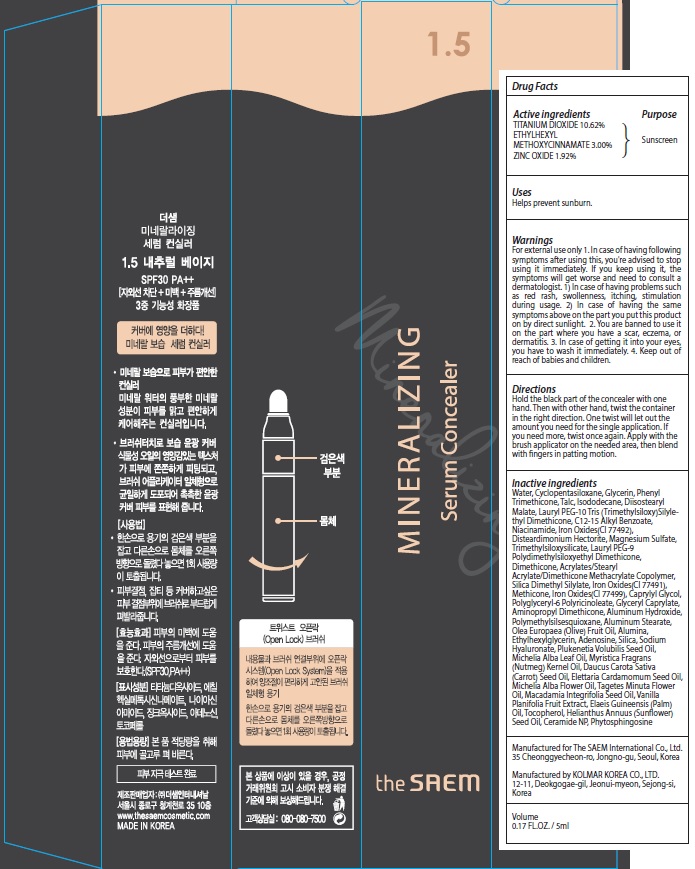
Mineralizing Serum Concealer 1.5 Natural Beige by The Saem International Co., Ltd. / Kolmar Korea Co., Ltd.
Mineralizing Serum Concealer 1.5 Natural Beige by
Drug Labeling and Warnings
Mineralizing Serum Concealer 1.5 Natural Beige by is a Otc medication manufactured, distributed, or labeled by The Saem International Co., Ltd., Kolmar Korea Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MINERALIZING SERUM CONCEALER 1.5 NATURAL BEIGE- titanium dioxide, octinoxate, zinc oxide cream
The Saem International Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ACTIVE INGREDIENT
Active ingredients: TITANIUM DIOXIDE 10.62%, ETHYLHEXYL METHOXYCINNAMATE 3.00%, ZINC OXIDE 1.92%
INACTIVE INGREDIENT
Inactive ingredients:
Water, Cyclopentasiloxane, Glycerin, Phenyl Trimethicone, Talc, Isododecane, Diisostearyl Malate, Lauryl PEG-10 Tris (Trimethylsiloxy)Silylethyl Dimethicone, C12-15 Alkyl Benzoate, Niacinamide, Iron Oxides(CI 77492), Disteardimonium Hectorite, Magnesium Sulfate, Trimethylsiloxysilicate, Lauryl PEG-9 Polydimethylsiloxyethyl Dimethicone, Dimethicone, Acrylates/Stearyl Acrylate/Dimethicone Methacrylate Copolymer, Silica Dimethyl Silylate, Iron Oxides(CI 77491), Methicone, Iron Oxides(CI 77499), Caprylyl Glycol, Polyglyceryl-6 Polyricinoleate, Glyceryl Caprylate, Aminopropyl Dimethicone, Aluminum Hydroxide, Polymethylsilsesquioxane, Aluminum Stearate, Olea Europaea (Olive) Fruit Oil, Alumina, Ethylhexylglycerin, Adenosine, Silica, Sodium Hyaluronate, Plukenetia Volubilis Seed Oil, Michelia Alba Leaf Oil, Myristica Fragrans (Nutmeg) Kernel Oil, Daucus Carota Sativa (Carrot) Seed Oil, Elettaria Cardamomum Seed Oil, Michelia Alba Flower Oil, Tagetes Minuta Flower Oil, Macadamia Integrifolia Seed Oil, Vanilla Planifolia Fruit Extract, Elaeis Guineensis (Palm) Oil, Tocopherol, Helianthus Annuus (Sunflower) Seed Oil, Ceramide NP, Phytosphingosine
WARNINGS
Warnings:
For external use only
1. In case of having following symptoms after using this, you're advised to stop using it immediately. If you keep using it, the symptoms will get worse and need to consult a dermatologist.
1) In case of having problems such as red rash, swollenness, itching, stimulation during usage.
2) In case of having the same symptoms above on the part you put this product on by direct sunlight.
2. You are banned to use it on the part where you have a scar, eczema, or dermatitis.
3. In case of getting it into your eyes, you have to wash it immediately.
4. Keep out of reach of babies and children.
DESCRIPTION
Uses:
Helps prevent sunburn.
Directions:
Hold the black part of the concealer with one hand. Then with other hand, twist the container in the right direction. One twist will let out the amount you need for the single application. If you need more, twist once again. Apply with the brush applicator on the needed area, then blend with fingers in patting motion.
| MINERALIZING SERUM CONCEALER 1.5 NATURAL BEIGE
titanium dioxide, octinoxate, zinc oxide cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - The Saem International Co., Ltd. (689402402) |
| Registrant - The Saem International Co., Ltd. (689402402) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Kolmar Korea Co.,LTD. Gwanjeong Factory | 689512611 | manufacture(70341-605) | |