GLATIRAMER ACETATE injection, solution
GLATIRAMER ACETATE by
Drug Labeling and Warnings
GLATIRAMER ACETATE by is a Prescription medication manufactured, distributed, or labeled by Italfarmaco SpA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
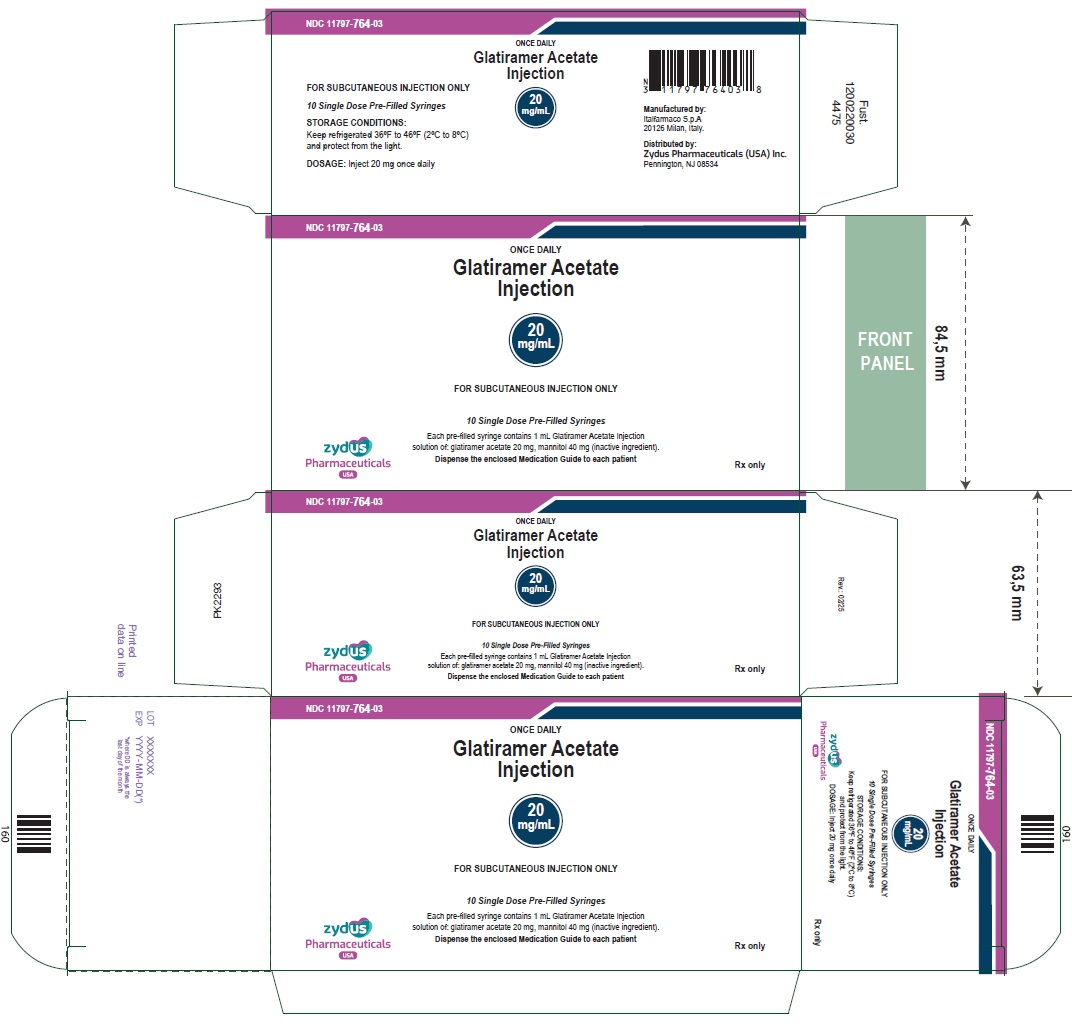
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 11797-764-03
ONCE DAILY
Glatiramer Acetate
Injection20
mg/mLFOR SUBCUTANEOUS INJECTION ONLY
30 pre-filled syringe (3 carton box of 10 syringes)
Each pre-filled syringe contains 1 mL Glatiramer Acetate Injection
solution of: glatiramer acetate 20 mg, mannitol 40 mg (inactive ingredient).
Dispense the enclosed Medication Guide to each patientzydus
Pharmaceuticals
USARx only
NDC: 11797-765-03
THREE TIMES A WEEK
Glatiramer Acetate
Injection40
mg/mLFOR SUBCUTANEOUS INJECTION ONLY
12 Single Dose Pre-Filled Syringes
Each pre-filled syringe contains 1 mL Glatiramer Acetate Injection
solution of: glatiramer acetate 40 mg, mannitol 40 mg (inactive ingredient).
Dispense the enclosed Medication Guide to each patientzydus
Pharmaceuticals
USARx only

-
INGREDIENTS AND APPEARANCE
GLATIRAMER ACETATE
glatiramer acetate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 11797-764 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLATIRAMER ACETATE (UNII: 5M691HL4BO) (GLATIRAMER - UNII:U782C039QP) GLATIRAMER ACETATE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) 40 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11797-764-03 30 in 1 CARTON 11/10/2025 1 1 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA208468 11/10/2025 GLATIRAMER ACETATE
glatiramer acetate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 11797-765 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLATIRAMER ACETATE (UNII: 5M691HL4BO) (GLATIRAMER - UNII:U782C039QP) GLATIRAMER ACETATE 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) 40 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11797-765-03 12 in 1 CARTON 11/10/2025 1 1 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA208468 11/10/2025 Labeler - Italfarmaco SpA (428179329) Establishment Name Address ID/FEI Business Operations Italfarmaco SpA 428179329 MANUFACTURE(11797-764, 11797-765) , ANALYSIS(11797-764, 11797-765) , PACK(11797-764, 11797-765)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.