PHENYLEPHRINE HYDROCHLORIDE solution/ drops
PHENYLEPHRINE HYDROCHLORIDE by
Drug Labeling and Warnings
PHENYLEPHRINE HYDROCHLORIDE by is a Prescription medication manufactured, distributed, or labeled by Caplin Steriles Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use
PHENYLEPHRINE HYDROCHLORIDE OPHTHALMIC SOLUTION, USP safely and effectively. See full prescribing information for PHENYLEPHRINE HYDROCHLORIDE OPHTHALMIC SOLUTION, USP.
PHENYLEPHRINE HYDROCHLORIDE ophthalmic solution, USP 2.5% and 10%.
Initial U.S. Approval: 1939
INDICATIONS AND USAGE
Phenylephrine Hydrochloride Ophthalmic Solution is an alpha-1 adrenergic receptor agonist indicated to dilate the pupil (1)
DOSAGE AND ADMINISTRATION
For patients 1 year of age and older: (2.1)
- Apply one drop of Phenylephrine Hydrochloride Ophthalmic Solution (2.5% or 10% strength) to conjunctival fornix at 3 to 5 minute intervals up to a maximum of 3 drops per eye.
- To obtain a greater degree of mydriasis, use 10% strength
For pediatric patients less than 1 year of age: (2.2)
- Instill one drop of 2.5% strength to conjunctival fornix at 3 to 5 minute intervals up to a maximum of 3 drops per eye
DOSAGE FORMS AND STRENGTHS
Ophthalmic solution (sterile): (3)
- 25 mg of phenylephrine hydrochloride in one mL of solution (2.5%)
- 100 mg of phenylephrine hydrochloride in one mL of solution (10%)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Not for injection: Topical ophthalmic use only (5.1)
- Serious cardiovascular reactions with 10% strength: Reactions have included ventricular arrhythmias and some have been fatal. Monitor blood pressure in patients with cardiovascular disease (5.2).
- Significant elevations in blood pressure: Caution in pediatric patients less than 5 years of age, and in patients with cardiovascular disease or hyperthyroidism. In patients at high risk, monitor blood pressure post treatment (5.3).
- Rebound miosis: Reported one day after instillation (5.4)
ADVERSE REACTIONS
- Ocular adverse reactions include eye pain and stinging on instillation, temporary blurred vision, and photophobia (6.1)
- Cardiovascular adverse reactions include increase in blood pressure, syncope, myocardial infarction, tachycardia, arrhythmia and subarachnoid hemorrhage (6.2)
To report SUSPECTED ADVERSE REACTIONS, contact Caplin Steriles Limited at 1-866-978-6111 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Recommendations
2.2 Dosing in Pediatric Patients Less Than 1 Year of Age
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Cardiac and Endocrine Disease
4.2 Pediatric Patients Less Than 1 Year of Age
5 WARNINGS AND PRECAUTIONS
5.1 Topical Ophthalmic Use Only
5.2 Cardiovascular Reactions
5.3 Elevation of Blood Pressure
5.4. Rebound Miosis
6 ADVERSE REACTIONS
6.1 Ocular Adverse Reactions
6.2 Systemic Adverse Reactions
7 DRUG INTERACTIONS
7.1 Agents That May Exaggerate Pressor Responses
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Recommendations
In patients 1 year of age or greater, apply one drop of either phenylephrine hydrochloride ophthalmic solution 2.5% or 10% every 3 to 5 minutes to the conjunctival fornix as required up to a maximum of 3 drops per eye per day.
In order to obtain a greater degree of mydriasis, phenylephrine hydrochloride ophthalmic solution 10% may be needed. -
3 DOSAGE FORMS AND STRENGTHS
Phenylephrine hydrochloride ophthalmic solution, USP 2.5% is a clear, colorless to yellow solution, sterile topical ophthalmic solution containing phenylephrine hydrochloride 2.5%: each mL contains 25 mg of phenylephrine hydrochloride.
Phenylephrine hydrochloride ophthalmic solution, USP 10% is a clear, colorless to yellow solution, sterile topical ophthalmic solution containing phenylephrine hydrochloride 10%.: each mL contains 100 mg of phenylephrine hydrochloride.
-
4 CONTRAINDICATIONS
4.1 Cardiac and Endocrine Disease
Phenylephrine hydrochloride ophthalmic solution 10% is contraindicated in patients with hypertension or thyrotoxicosis. Phenylephrine hydrochloride ophthalmic solution 2.5% should be used in these patients.
4.2 Pediatric Patients Less Than 1 Year of Age
Phenylephrine hydrochloride ophthalmic solution 10% is contraindicated in pediatric patients less than 1 year of age due to the increased risk of systemic toxicity. Phenylephrine hydrochloride ophthalmic solution 2.5% should be used in these patients [See Dosage and Administration (2.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Topical Ophthalmic Use Only
Phenylephrine hydrochloride ophthalmic solution 2.5% and 10% is not indicated for injection.
5.2 Cardiovascular Reactions
There have been reports of serious cardiovascular reactions, including ventricular arrhythmias and myocardial infarctions, in patients using phenylephrine 10%. These episodes, some fatal, have usually occurred in patients with pre-existing cardiovascular diseases. Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% should be used in these patients.
5.3 Elevation of Blood Pressure
A significant elevation in blood pressure is not common but has been reported following conjunctival instillation of recommended doses of phenylephrine 10%. The risk is less with phenylephrine 2.5%. Caution should be exercised with the use of phenylephrine 10% in pediatric patients less than 5 years of age and patients with hyperthyroidism, or cardiovascular disease. The post-treatment blood pressure of patients with cardiac and endocrine diseases and any patients who develop symptoms should be carefully monitored.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in the labeling:
- Cardiovascular Effects [See Warnings and Precautions (5.2)]
- Elevation in Blood Pressure [See Warnings and Precautions (5.3)]
The following adverse reactions have been identified following use of phenylephrine hydrochloride ophthalmic solution. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
6.1 Ocular Adverse Reactions
Eye pain and stinging on instillation, temporary blurred vision and photophobia, and conjunctival sensitization may occur.
6.2 Systemic Adverse Reactions
A marked increase in blood pressure has been reported particularly, but not limited to low weight premature neonates, infants and hypertensive patients.
Cardiovascular effects which have been seen primarily in hypertensive patients following topical ocular use of phenylephrine hydrochloride ophthalmic solution 10% include marked increase in blood pressure, syncope, myocardial infarction, tachycardia, arrhythmia and subarachnoid hemorrhage [See Warnings and Precautions (5.2 and 5.3)].
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Animal reproduction studies have not been conducted with topical phenylephrine. It is also not known whether phenylephrine can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Phenylephrine hydrochloride should be given to a pregnant woman only if clearly needed.
8.3 Nursing Mothers
It is not known whether this drug is excreted in human breast milk. Because many drugs are excreted in human milk, caution should be exercised when phenylephrine hydrochloride ophthalmic solution 2.5% and 10% is administered to a nursing woman.
8.4 Pediatric Use
Phenylephrine hydrochloride ophthalmic solution 10% is contraindicated in pediatric patients less than 1 year of age [See Contraindications (4.2)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
Phenylephrine Hydrochloride Ophthalmic Solution, USP is a sterile, clear, colorless, topical α- adrenergic agonist for ophthalmic use. The active ingredient is represented by the chemical structure
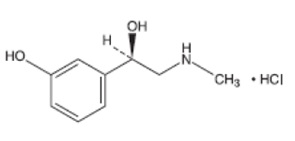
Chemical Name: (R)-3-hydroxy-α[(methylamino)methyl]benzenemethanol hydrochloride.
Molecular Formula: C9H13NO2.HCl
Molecular Weight: 203.67 g/mol
Each mL of Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% contains: ACTIVE: Phenylephrine Hydrochloride 25 mg (2.5%); INACTIVES: Sodium Phosphate Dibasic, Sodium Phosphate Monobasic, Water for Injection. Phosphoric Acid and/or Sodium Hydroxide may be added to adjust pH (4.0 to 7.5). The solution has a tonicity of 340 mOsm/kg; PRESERVATIVE: Benzalkonium Chloride 0.1 mg (0.01%).
Each mL of Phenylephrine Hydrochloride Ophthalmic Solution, USP 10% contains: ACTIVE: Phenylephrine Hydrochloride 100 mg (10%); INACTIVES: Sodium Phosphate Dibasic, Sodium Phosphate Monobasic, Water for Injection. Phosphoric Acid and/or Sodium Hydroxide may be added to adjust pH (4.0 to 7.5). The solution has a tonicity of 985 mOsm/kg; PRESERVATIVE: Benzalkonium Chloride 0.1 mg (0.01%). -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Phenylephrine hydrochloride is a α-1 adrenergic agonist drug that is used in ophthalmology mainly for its mydriatic effect. After topical application to the conjunctiva, phenylephrine acts directly on α-adrenergic receptors in the eye, producing contraction of the dilator muscle of the pupil and constriction of the arterioles in the conjunctiva.
12.2 Pharmacodynamics
Maximal mydriasis occurs in 20 to 90 minutes with recovery after 3 to 8 hours.
Systemic absorption of sufficient quantities of phenylephrine may lead to systemic α-adrenergic effects, such as rise in blood pressure which may be accompanied by a reflex atropine-sensitive bradycardia.
-
14 CLINICAL STUDIES
Pupillary dilation following topical administration of phenylephrine hydrochloride ophthalmic solution has been demonstrated in controlled clinical studies in adults and pediatric patients with different levels of iris pigmentation. Pupil movement is generally seen within 15 minutes, maximal mydriasis between 20 to 90 minutes and recovery after 3 to 8 hours. Darker irides tend to dilate slower than lighter irides.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Phenylephrine Hydrochloride Ophthalmic Solution, USP 2.5% is supplied as a sterile, aqueous, topical ophthalmic solution in an opaque, white low density polyethylene (LDPE) bottle with a natural LDPE dropper tip and red cap in the following sizes:
NDC: 65145-148-01 2 mL in 5 mL bottle
NDC: 65145-149-01 15 mL in 15 mL bottle
Phenylephrine Hydrochloride Ophthalmic Solution, USP 10% is supplied as a sterile, aqueous, topical ophthalmic solution in an opaque, white low density polyethylene (LDPE) bottle with a natural LDPE dropper tip and red cap in the following sizes:
NDC: 65145-150-01 5 mL in 10 mL bottle
After opening, Phenylephrine Hydrochloride Ophthalmic Solution, USP can be used until the expiration date on the bottle.
Storage: Store at 20° to 25°C (68° to 77°F).
Keep container tightly closed.
Protect from light and excessive heat.Do not use if solution is brown or contains precipitate.
-
17 PATIENT COUNSELING INFORMATION
Advise patients not to touch the dropper tip to any surface as this may contaminate the solution. Inform patients that they may experience sensitivity to light and should protect their eyes in bright illumination while their pupils are dilated.
Made in India

Distributed by :
Caplin Steriles USA Inc,
Hamilton, NJ 08619.
Code: TN/Drugs/TN00003457
Revised date: 08/2024
22200945 -
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Container Label - 2mL
NDC 65145-148-01
Phenylephrine Hydrochloride
Ophthalmic Solution, USP
2.5%
For eye use only
Rx only 2 mL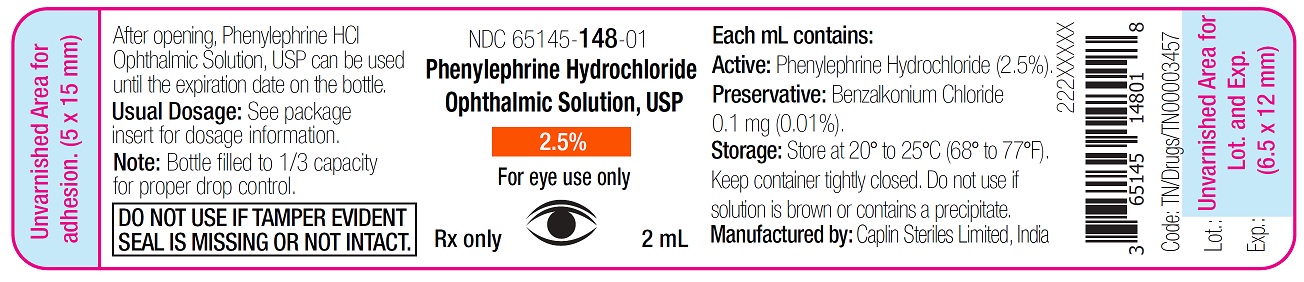
Carton Label - 2 mL
NDC 65145-148-01
Phenylephrine
Hydrochloride
Ophthalmic
Solution, USP
2.5%
For eye use only
Rx only 2 mL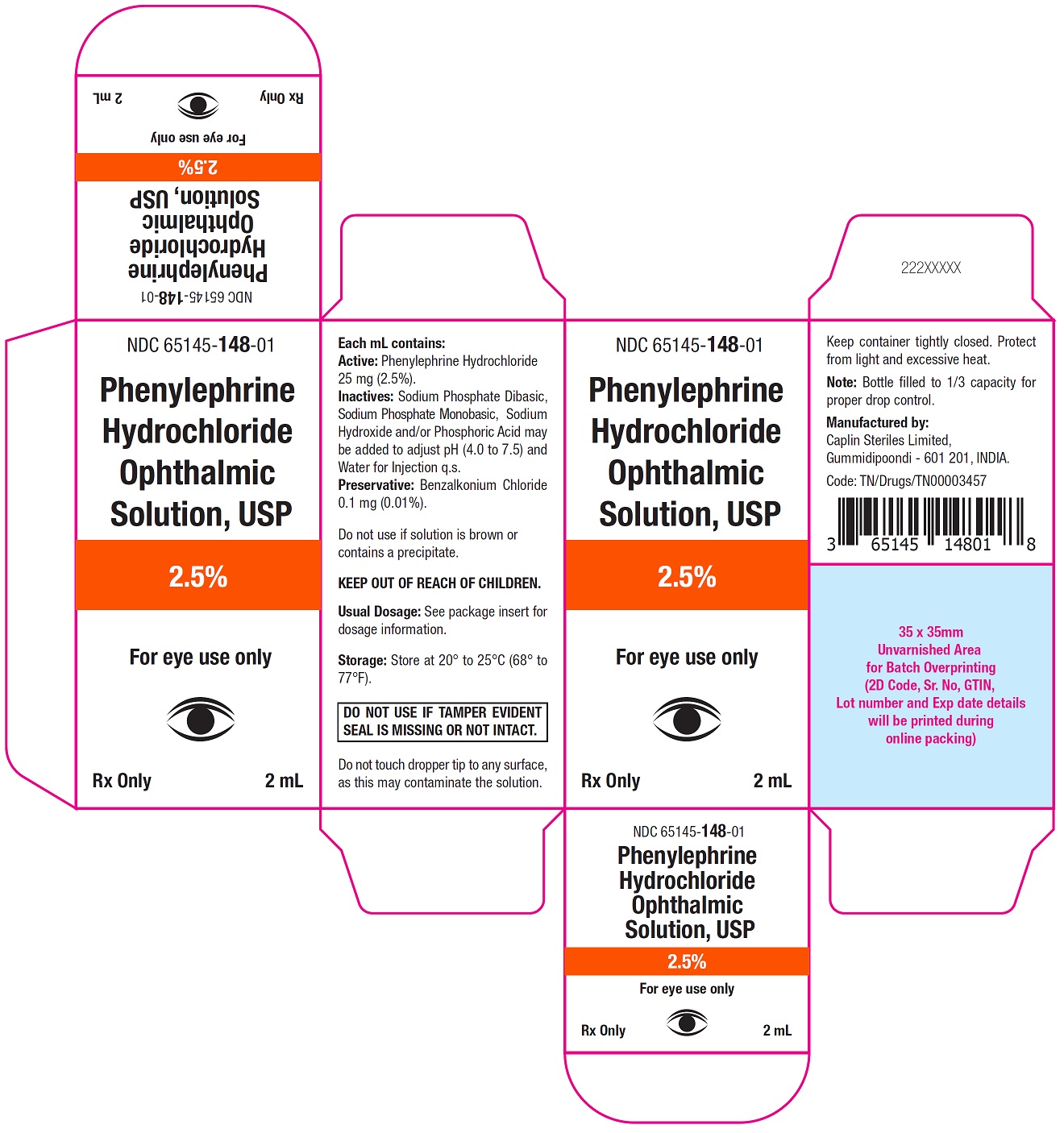
Container Label - 15 mL
NDC 65145-149-01
Phenylephrine Hydrochloride
Ophthalmic Solution, USP
2.5%
For eye use only
Rx only 15 mL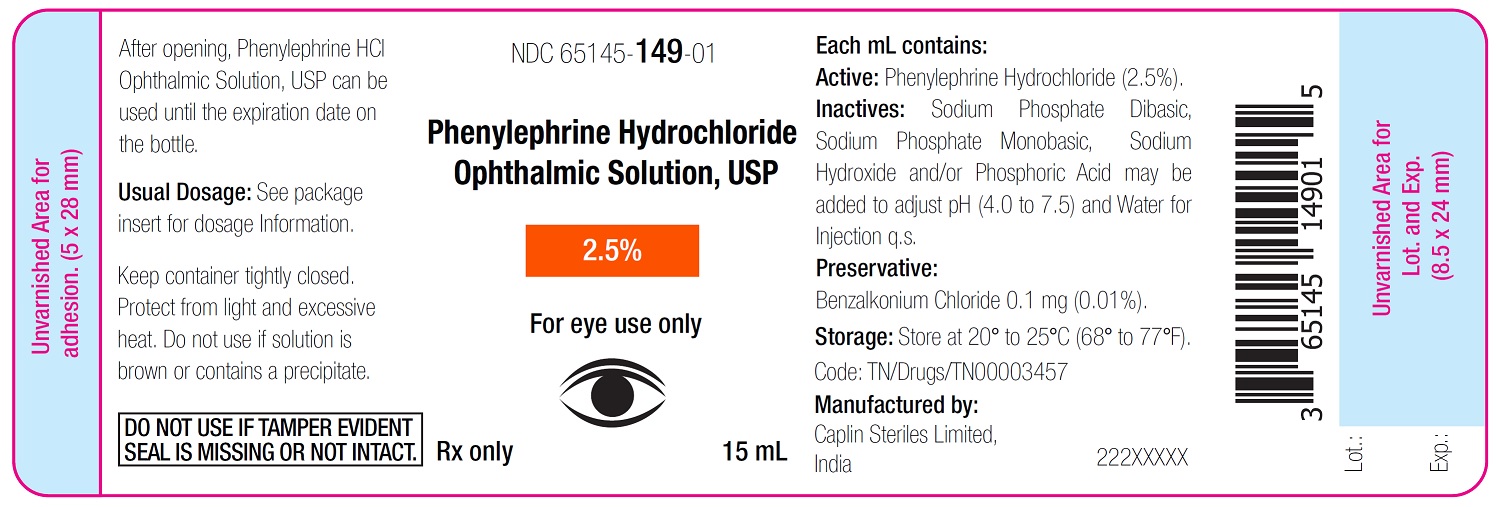
Carton Label - 15 mL
NDC 65145-149-01
Phenylephrine
Hydrochloride
Ophthalmic
Solution, USP
2.5%
For eye use only
Rx only 15 mL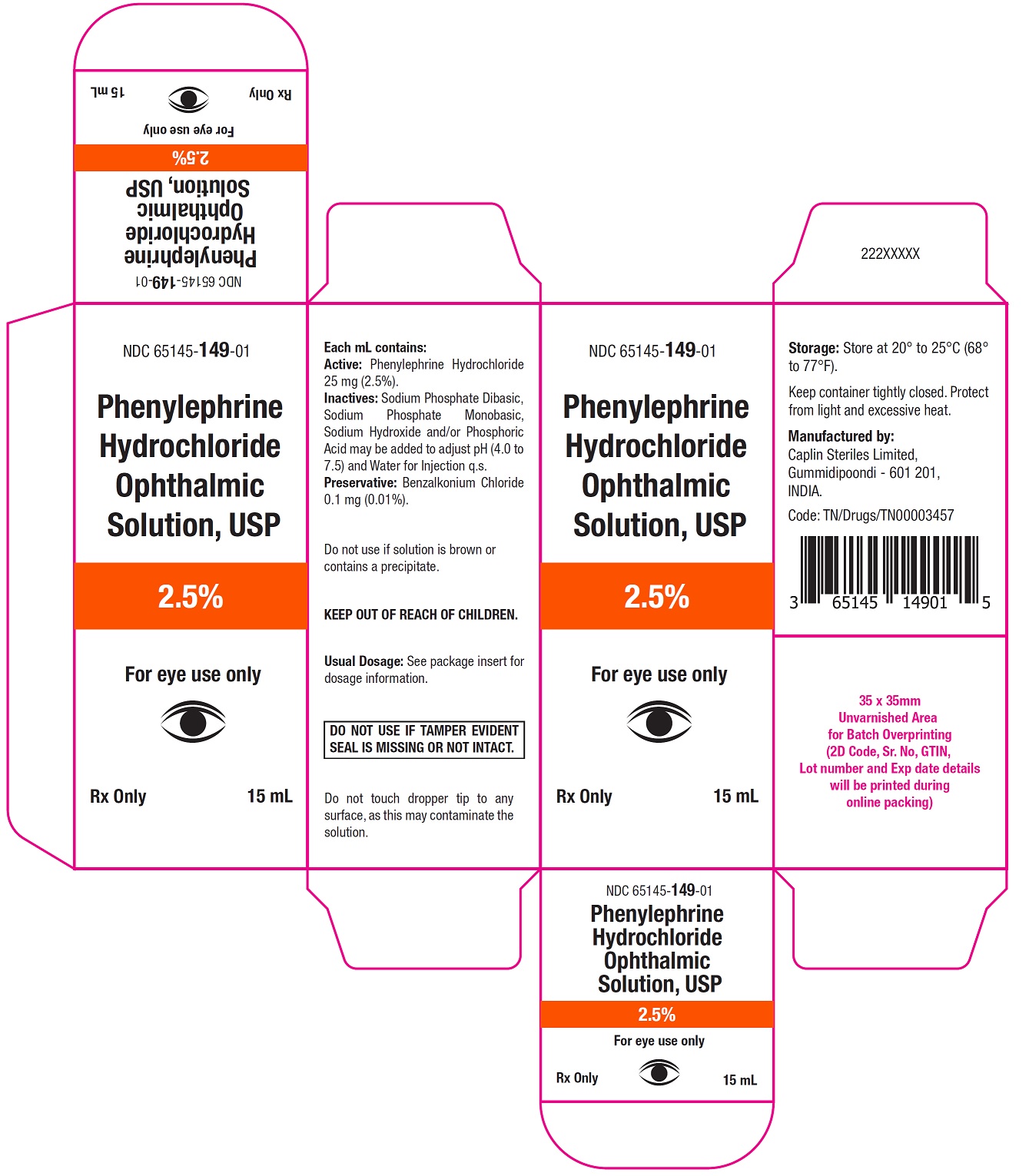
Container Label - 5 mL
NDC 65145-150-01
Phenylephrine Hydrochloride
Ophthalmic Solution, USP
10%
For eye use only
Rx only 5 mL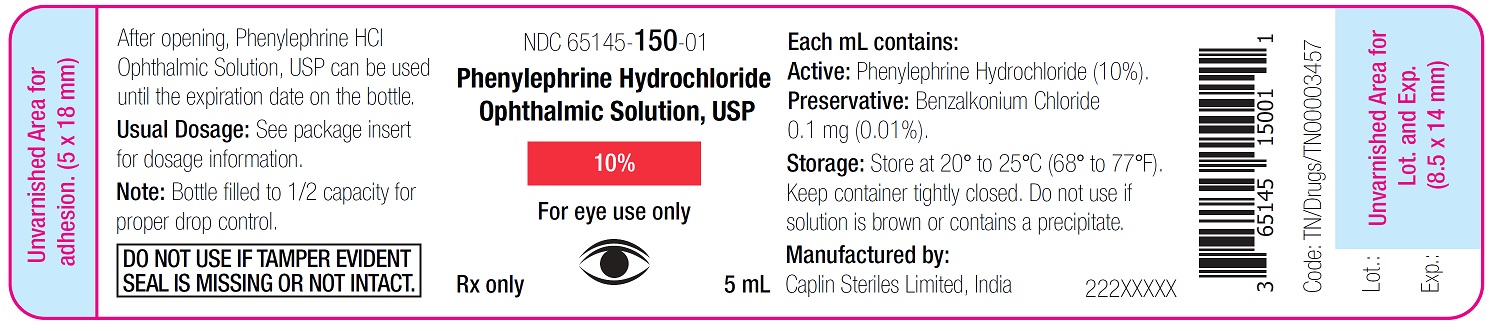
Carton Label - 5 mL
NDC 65145-150-01
Phenylephrine
Hydrochloride
Ophthalmic
Solution, USP
10%
For eye use only
Rx only 5 mL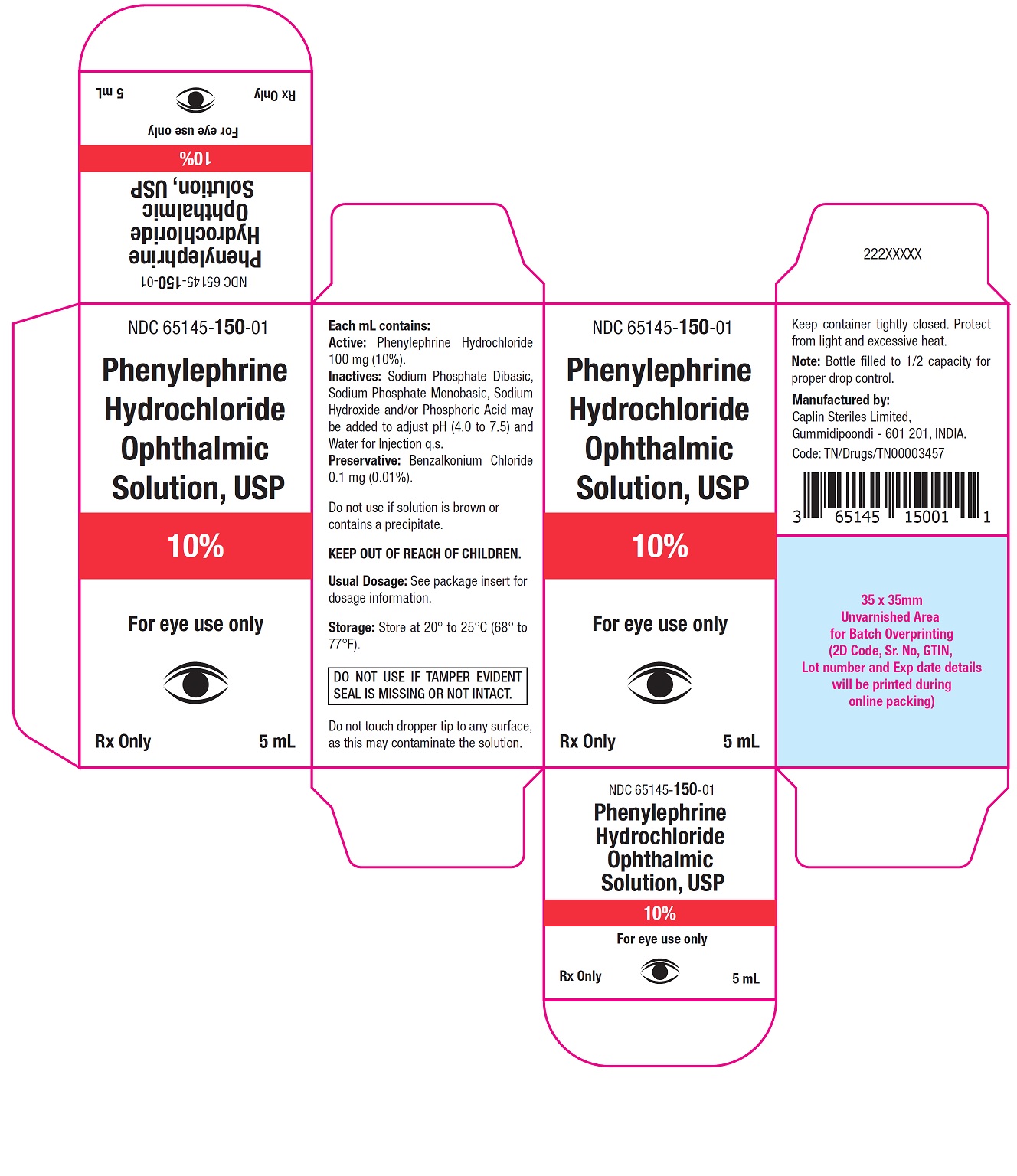
-
INGREDIENTS AND APPEARANCE
PHENYLEPHRINE HYDROCHLORIDE
phenylephrine hydrochloride solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65145-148 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 25 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SODIUM HYDROXIDE (UNII: 55X04QC32I) PHOSPHORIC ACID (UNII: E4GA8884NN) Benzalkonium Chloride (UNII: F5UM2KM3W7) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65145-148-01 1 in 1 CARTON 01/23/2025 1 2 mL in 1 BOTTLE, DROPPER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215183 01/23/2025 PHENYLEPHRINE HYDROCHLORIDE
phenylephrine hydrochloride solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65145-149 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 25 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SODIUM HYDROXIDE (UNII: 55X04QC32I) PHOSPHORIC ACID (UNII: E4GA8884NN) Benzalkonium Chloride (UNII: F5UM2KM3W7) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65145-149-01 1 in 1 CARTON 01/23/2025 1 15 mL in 1 BOTTLE, DROPPER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215183 01/23/2025 PHENYLEPHRINE HYDROCHLORIDE
phenylephrine hydrochloride solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 65145-150 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM PHOSPHATE, DIBASIC, HEPTAHYDRATE (UNII: 70WT22SF4B) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) SODIUM HYDROXIDE (UNII: 55X04QC32I) PHOSPHORIC ACID (UNII: E4GA8884NN) Benzalkonium Chloride (UNII: F5UM2KM3W7) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65145-150-01 1 in 1 CARTON 01/23/2025 1 5 mL in 1 BOTTLE, DROPPER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215183 01/23/2025 Labeler - Caplin Steriles Limited (650744670) Establishment Name Address ID/FEI Business Operations Caplin Steriles Limited 650744670 ANALYSIS(65145-148, 65145-149, 65145-150) , MANUFACTURE(65145-148, 65145-149, 65145-150) , PACK(65145-148, 65145-149, 65145-150)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.