FESILTY- fibrinogen, human-chmt kit
FESILTY by
Drug Labeling and Warnings
FESILTY by is a Other medication manufactured, distributed, or labeled by Grifols USA LLC, Biotest AG, Grifols Therapeutics LLC, Laboratorios Grifols, S.A., Instituto Grifols, S.A., Prothya Biosolutions Belgium, TechPharm GmbH, GBA Pharma GmbH. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use FESILTY safely and effectively. See full prescribing information for FESILTY.
FESILTY (fibrinogen, human - chmt), lyophilized powder for reconstitution, for intravenous use
Initial U.S. Approval: 2025INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
Intravenous use after reconstitution only.
- Calculate the dose in mg fibrinogen per kg of BW for each patient individually. The target plasma fibrinogen level is 100 mg/dL for minor bleeding and 150 mg/dL for major bleeding. (2.1)
When plasma fibrinogen level is known:
Patient Age Calculation for Recommended Dose (mg/kg BW) Adults and pediatric patients ≥ 6 years of age [Target fibrinogen level (mg/dL) – Measured fibrinogen level (mg/dL)] /1.8 (mg/dL per mg/kg BW) Pediatric patients < 6 years of age [Target fibrinogen level (mg/dL) – Measured fibrinogen level (mg/dL)] /1.6 (mg/dL per mg/kg BW) When plasma fibrinogen is not known:
Dose: 70 mg/kg BW for patients of all ages
Frequency and duration of dosing:
- Monitor fibrinogen levels. Individualize the frequency and duration of dosing based on the extent of bleeding, plasma fibrinogen level, and the clinical condition of the patient.
- Infuse FESILTY using an infusion pump at an infusion rate not to exceed 5 mL/min. Initial infusion rates are: (2.3)
Patient Age Maximum Infusion Rate Adults and pediatric patients ≥ 6 years of age
5 mL/min Pediatric patients 4 to < 6 years of age
1.0 mL/min Pediatric patients 2 to < 4 years of age
0.75 mL/min Pediatric patients 28 days to < 2 years of age
0.30 mL/min Newborns (0 to 27 days)
0.10 mL/min DOSAGE FORMS AND STRENGTHS
FESILTY is a sterile, lyophilized, white in color powder for solution for intravenous injection. FESILTY is provided as one single-dose glass vial containing nominally 1 gram of human fibrinogen and one 50 mL glass vial of Sterile Water for Injection, USP. The actual amount of fibrinogen in milligrams fibrinogen per vial is printed on the vial label and carton. (3)
CONTRAINDICATIONS
Severe hypersensitivity reactions, including anaphylaxis, to FESILTY or its components (arginine hydrochloride, polysorbate 80, sodium citrate dihydrate, trehalose dihydrate) (4)
WARNINGS AND PRECAUTIONS
- Hypersensitivity reactions have occurred in patients receiving FESILTY. Should symptoms occur, discontinue FESILTY and administer appropriate treatment. (5.1)
- Thrombotic events have been reported in patients receiving FESILTY. Weigh the benefits of administration versus the risks of thrombosis. (5.2)
- FESILTY is made from pooled human plasma and may carry the risk of transmitting infectious agents. All infections thought to be transmitted by FESILTY should be reported to Grifols at 1-800-520-2807, (5.3)
ADVERSE REACTIONS
The most common adverse reactions (incidence > 2%) were pain in extremity, back pain, hypersensitivity reactions, pyrexia, thrombosis, fibrin D dimer increased, headache, and vomiting. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Grifols Therapeutics LLC at 1-800-520-2807 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.Revised: 12/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dose
2.2 Preparation and Reconstitution
2.3 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
5.2 Thrombosis
5.3 Transmissible Infectious Agents
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dose
For intravenous use after reconstitution only
The target plasma fibrinogen level is 100 mg/dL for minor bleeding and 150 mg/dL for major bleeding.
The actual amount of fibrinogen in milligrams per vial of FESILTY is printed on the vial label and carton.
FESILTY dose when baseline fibrinogen level is known
The dose for each patient must be individually calculated taking into consideration age, the location and extent of bleeding, the plasma level of fibrinogen (mg/dL), and the clinical condition of the patient.
Calculate the recommended dose in milligrams fibrinogen per kilogram BW according to the age of the patient:
- Adults and pediatric patients ≥ 6 years of age
Dose (mg/kg BW) = Target fibrinogen level (mg/dL) – Measured fibrinogen level (mg/dL) 1.8 (mg/dL per mg/kg BW) - Pediatric patients < 6 years of age
Dose (mg/kg BW) = Target fibrinogen level (mg/dL) – Measured fibrinogen level (mg/dL) 1.6 (mg/dL per mg/kg BW) Monitor plasma fibrinogen level and repeat the dose if the plasma fibrinogen level drops below the target level until hemostasis is achieved.
Individualize the frequency and duration of dosing based on the extent of bleeding, plasma fibrinogen level, and the clinical condition of the patient.
FESILTY dose when baseline fibrinogen level is not known
The recommended dose when the baseline fibrinogen is not known is 70 mg/kg BW for patients of all ages.
Monitor plasma fibrinogen level and repeat the dose as needed to maintain the target fibrinogen level.
Individualize the frequency and duration of dosing based on the extent of bleeding, plasma fibrinogen level, and the clinical condition of the patient.2.2 Preparation and Reconstitution
FESILTY is a white powder. Upon reconstitution with sterile water, the solution is almost colorless and clear to slightly opalescent.
The following procedures are provided as a guide for the preparation and reconstitution of FESILTY.
Preparation
Inspect the carton kit before opening. Discard the kit if the package is damaged or if the seal on the carton shows signs of tampering.
Do not use FESILTY after the expiration date printed on the vial label and carton.
If stored refrigerated, allow the unopened vials of Sterile Water for Injection, USP (vial number 1) and product (FESILTY, vial number 2) to come to room temperature.
Use aseptic technique (clean and sanitized) and a flat surface during reconstitution of FESILTY.
Reconstitution with nextaro® v, 20/20 5 µm transfer system- Remove the caps from the water vial and the product vial in order to expose the central portions of the stoppers (Fig. 1). Cleanse each vial stopper with an alcohol swab and allow surface to dry.
- Completely remove the paper seal of the transfer system blister package (Fig. 2). To maintain sterility, keep the transfer device in the clear blister package. Do not touch the spike.
- Place the water vial on an even surface. Place the blue part of the transfer system within the blister straight onto the upright water vial (Fig. 3) until it snaps into place. Do not twist the transfer system.
- Remove the clear part of the blister package from the transfer system. Now the white part of the transfer system is visible (Fig. 4).
- Place the product vial on an even surface.
- Turn the combination of transfer system and water vial upside down (Fig. 5). Push the spike of the white end of the transfer system straight down through the product vial stopper (Fig. 6) until it snaps into place. The vacuum present in the product vial causes the water to flow into the product vial (Fig. 7). Wait until water transfer is complete.
- Gently sway the unit consisting of the transfer system, product and water vial, to dissolve the powder. Do not shake the unit, to avoid foaming. The powder should be dissolved completely within approximately 3 minutes. Discard the product if the powder is not fully dissolved within 30 minutes. After reconstitution the solution should be clear or slightly opalescent.
- Afterwards unscrew the blue part of the transfer system together with the empty water vial counterclockwise (Fig. 8). Discard the water vial with the blue part of the transfer system attached. The luer-lock connector is now visible. To maintain sterility do not touch the luer-lock connector.
- The solution is ready for use. Keep the solution at room temperature and use within 4 hours after dissolving. Do not use solutions that are cloudy or contain visible particles.
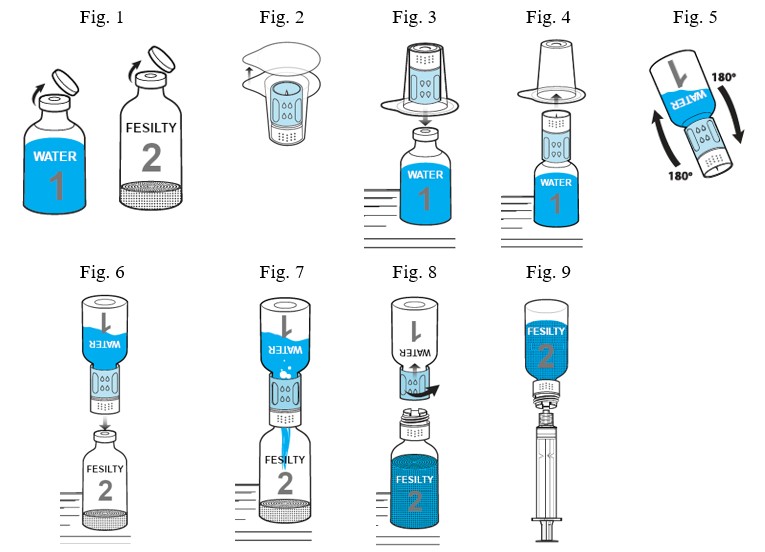
- Screw a sterile syringe (not supplied) onto the luer-lock connector of the product vial with the white part of the transfer system and invert (Fig. 9) to allow you to easily draw the dissolved drug into the syringe. Use constant force during drawing to avoid foaming. A separate filter is not necessary because the transfer system has its own integrated filter.
- Carefully disconnect the vial with the white part of the transfer system from the syringe. A standard infusion set is recommended for intravenous injection of the solution.
- If the dose requires more than one vial of FESILTY, reconstitute each vial using a new nextaro® v, 20/20 transfer system provided in the carton. The nextaro® v, 20/20 transfer system is for single use only.
Do not shake.
Do not mix FESILTY with other intravenous medications. Administer by a separate injection/infusion line.
FESILTY is for single use only. It contains no preservatives.
Discard unused portion.2.3 Administration
Inspect visually for particulate matter and discoloration prior to administration. Do not use if the liquid is cloudy or turbid, discolored, or if it contains visible particulate matter.
Infusion Rates
Infuse FESILTY intravenously using an infusion pump at an infusion rate not to exceed 5 mL/min. The initial infusion rates are provided in Table 1. Selection of the infusion rate remains principally at the discretion of the treating physician considering the exact clinical situation of the patient.Table 1: Infusion Rates for FESILTY Patient Age Initial Infusion Rate* - * Infusion rate not to exceed 5 mL/min
Adults and pediatric patients ≥ 6 years of age 5 mL/min Pediatric patients 4 to < 6 years of age 1.0 mL/min Pediatric patients 2 to < 4 years of age 0.75 mL/min Pediatric patients 28 days to < 2 years of age 0.30 mL/min Newborns (0 to 27 days) 0.10 mL/min -
3 DOSAGE FORMS AND STRENGTHS
FESILTY is a sterile, lyophilized, white in color powder for solution for intravenous injection. FESILTY is provided as one single-dose glass vial containing nominally 1 gram of human fibrinogen and one 50 mL glass vial of Sterile Water for Injection, USP. The actual amount of fibrinogen in milligrams of fibrinogen per vial is printed on the vial label and carton.
-
4 CONTRAINDICATIONS
FESILTY is contraindicated in patients who have severe hypersensitivity reactions, including anaphylaxis, to FESILTY or its components (arginine hydrochloride, polysorbate 80, sodium citrate dihydrate, trehalose dihydrate). [see Warnings and Precautions (5.1)]
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity
Hypersensitivity reactions have occurred to FESILTY or its components (arginine hydrochloride, polysorbate 80, sodium citrate dihydrate, trehalose dihydrate). Monitor patients for signs and symptoms of hypersensitivity reactions including hives, generalized urticaria, tightness of the chest, wheezing, hypotension, and anaphylaxis. If symptoms occur, discontinue FESILTY infusion immediately. Manage patients based on clinical practice accordingly.
5.2 Thrombosis
Thrombotic events have occurred in patients receiving FESILTY [see Adverse Reactions (6)]. Thrombosis may occur spontaneously in patients with congenital fibrinogen deficiency with or without the use of fibrinogen replacement therapy. Weigh the benefits of FESILTY administration versus the risks of thrombosis. Monitor patients receiving FESILTY for signs and symptoms of thrombosis.
5.3 Transmissible Infectious Agents
Because FESILTY is made from pooled human plasma, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. This also applies to unknown or emerging viruses and other pathogens. The risk of infectious agent transmission has been reduced by screening plasma donors and by including virus inactivation as well as virus and prion removal steps in the manufacturing process of FESILTY[see Description (11)].
All infections suspected to have been transmitted by FESILTY should be reported by the physician or other healthcare provider to Grifols at 1-800-520-2807. -
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of one drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety data described in this section reflects exposure to FESILTY in one clinical study (Study 984). A total of 45 patients with congenital fibrinogen deficiency received at least one dose of FESILTY. A total of 175 bleeding episodes were treated with a mean dose of 3.9 g [see Clinical Studies (14)].
Serious adverse reactions occurred in 4 patients (9%) including portal vein thrombosis (n=1), deep vein thrombosis (n=1), and pain in extremity with clinically suspected thrombosis (n=1). One patient had episode of epilepsy and died due to extradural hematoma four weeks after FESILTY administration.
Table 2 lists the most common adverse reactions that occurred in > 2% patients in Study 984.Table 2: Adverse Reactions Occurring in > 2% Patients in Study 984 Adverse Reaction N=45
n (%)- * Hypersensitivity reactions = all patients had adverse reactions of facial swelling.
- † Thrombosis includes portal vein thrombosis and deep vein thrombosis.
Pain in extremity 3 (7) Back pain 3 (7) Hypersensitivity reactions* 3 (7) Pyrexia 2 (4) Thrombosis† 2 (4) Fibrin D dimer increased 2 (4) Headache 2 (4) Vomiting 2 (4) -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
No human data are available to indicate the presence or absence of a drug-associated risk. Animal reproduction studies have not been conducted with FESILTY. One patient in clinical trial 984 reported pregnancy 11 months after receiving FESILTY. The patient withdrew from the study and there were no reports of complications during the pregnancy. It is not known whether FESILTY can cause fetal harm when administered to a pregnant woman or can affect fertility.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20% respectively.8.2 Lactation
Risk Summary
There is no information regarding the presence of FESILTY in breast milk, the effect on the breast-fed infant, or the effects on milk production. The developmental and health benefits of breast feeding should be considered along with the mother’s clinical need for FESILTY and any potential adverse effects on the breast-fed infant from FESILTY or from the underlying maternal condition.8.4 Pediatric Use
The safety and effectiveness of FESILTY have been established in pediatric patients with congenital fibrinogen deficiency. Use of FESILTY in pediatric patients was supported by evidence from one clinical study (Study 984) that enrolled 24 pediatric patients 0 to 16 years of age (6 patients aged 0 to < 6 years, 18 patients aged ≥6 years) with congenital fibrinogen deficiency [see Adverse Reactions (6) and Clinical Studies (14)].
-
11 DESCRIPTION
FESILTY (fibrinogen, human – chmt), is a purified, sterile, non-pyrogenic, lyophilized powder of human fibrinogen for reconstitution for intravenous administration. Human fibrinogen is purified from Source Plasma from the cryoprecipitate fraction and processed using a combination of aluminum hydroxide purification, solvent/detergent (S/D) treatment, anion and cation exchange chromatography, glycine precipitation, and Ultraviolet (UV)-C irradiation. FESILTY is supplied in a single-dose vial containing nominally 1 gram of fibrinogen. The actual amount of fibrinogen is printed on the vial label and carton in milligrams fibrinogen per vial. When reconstituted with 50 mL sterile water for injection, FESILTY contains approximately 20 mg/mL protein, of which not less than 80% is fibrinogen monomer. Each vial of FESILTY also contains 421.3 mg arginine hydrochloride, 292.2 mg sodium chloride, 73.5 mg sodium citrate dihydrate, 25.5 mg polysorbate 80, and 567.5 mg trehalose dihydrate. FESILTY has a pH of 6.5 to 7.5 and an osmolality of ≥ 240 mOsmol/kg. FESILTY does not contain preservatives and is not made with natural rubber latex.
FESILTY is prepared from pooled Source Plasma obtained from healthy volunteer donors. Each plasma donation used for the manufacture of FESILTY is collected from FDA-licensed facilities. Plasma donations must test negative for hepatitis B virus (HBV) surface antigen (HBsAg), antibodies to human immunodeficiency virus (HIV) strains 1 and 2 (anti-HIV-1/2), and antibodies to the hepatitis C virus (anti-HCV) as determined by enzyme immunoassay (EIA). In addition, samples from manufacturing pools must test non-reactive for HIV RNA, HCV RNA, HBV DNA, and Hepatitis A Virus (HAV) RNA, by Nucleic Acid Amplification Testing (NAT). Parvovirus B19 (B19V) DNA is also tested by NAT and must not exceed 104 IU/mL in the manufacturing pool.
The manufacturing process for FESILTY employs several steps to remove/inactivate adventitious viruses to further increase the margins of safety. These steps include S/D treatment, UV-C irradiation, and heat treatment of lyophilized fibrinogen. Virus clearance studies with a scaled-down process have been performed for these steps to determine their capacity to inactivate or remove both enveloped and non-enveloped viruses. The results are shown in Table 3.Table 3: Virus Reduction Factors (log10) during FESILTY Manufacturing Process for Enveloped (E) and Non-enveloped (NE) Viruses - * HIV: Human immunodeficiency virus
- † PRV: Porcine pseudorabies virus (varicellovirus suidalpha 1), a generic model for hepatitis B virus
- ‡ BVDV: Bovine viral diarrhea virus 1 (pestivirus bovis), a model for hepatitis C virus (hepacivirus hominis)
- § HAV: Hepatitis A virus (hepatovirus A)
- ¶ PPV: Porcine parvovirus (protoparvovirus ungulate 1), a model for human parvovirus B19 (erythroparvovirus primate1)
- # S/D: Solvent/detergent
- Þ n.d.: Not determined
- ß UVC: Ultraviolet-C
Step HIV*(E) PRV†(E) BVDV‡(E) HAV§(NE) PPV¶(NE) S/D# Treatment ≥ 4.51 ≥ 5.39 ≥ 5.21 n.d.Þ n.d.Þ UV-Cß Irradiation n.d.Þ 1.63 1.87 2.47 4.19 Lyophilization & Dry Heat Treatment ≥ 4.86 ≥ 5.36 ≥ 4.29 ≥ 4.34 1.09 Total Clearance ≥ 9.37 ≥ 12.38 ≥ 11.37 ≥ 6.81 5.28 The manufacturing process was also investigated for its capacity to reduce the infectivity of an experimental agent of transmissible spongiform encephalopathy (TSE), considered a model for CJD and its variant, vCJD. One chromatographic purification step has been shown to reduce TSE infectivity of an experimental model agent. These studies provide reasonable assurance that low levels (at least 3.27 log10) of vCJD/CJD agent infectivity, if present in the starting material, would be removed.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fibrinogen (Factor I) is a soluble plasma protein that, during the coagulation process, is converted to fibrin, one of the key components of the blood clot. Fibrinogen is a heterohexamer with a molecular weight of 340 kDa and composed of two sets of alpha, beta, and gamma polypeptide chains.
Fibrinogen plays a fundamental role in achieving and maintaining overall hemostasis. Following coagulation activation and thrombin generation, fibrinogen is cleaved by thrombin at specific sites on the alpha and beta chains to remove fibrinopeptide A (FPA) and fibrinopeptide B (FPB). The removal of FPA and FPB exposes binding sites on the fibrinogen molecule and leads to the formation of fibrin monomers that subsequently undergo polymerization. The resulting fibrin is stabilized by activated factor XIII which forms cross links between fibrin polymers and renders the fibrin clot more resistant to fibrinolysis. Additionally, soluble fibrinogen mediates platelet aggregation by binding to the glycoprotein IIb/IIIa (GPIIb/IIIa) receptor on the platelet surface activated following blood vessel injury. This interaction acts as a bridge between platelets, facilitating their aggregation, i.e., formation of primary platelet plug. The end product of the coagulation cascade, cross-linked fibrin, stabilizes and reinforces the primary platelet plug to achieve secondary hemostasis and stop bleeding.
12.2 Pharmacodynamics
In a prospective, open-label, multicenter phase I clinical trial the pharmacodynamic properties for FESILTY were evaluated in 27 patients of all age groups with congenital afibrinogenemia or severe hypofibrinogenemia. The assessment was based on fibrinogen activity (FiAc) levels in plasma after a single intravenous administration of 70 mg/kg BW. Maximum clot firmness (MCF) was measured by thromboelastometry and showed a positive correlation with FiAc levels of FESILTY in patients across the age groups.
12.3 Pharmacokinetics
PK properties of FESILTY were investigated based on FiAc levels in plasma after a single intravenous administration of 70 mg/kg BW in patients with congenital afibrinogenemia or severe hypofibrinogenemia of all age groups.
Table 4: Summary of Pharmacokinetic Parameters for FESILTY Fibrinogen Activity (FiAc) by Age Groups Abbreviations: AUC0-∞ = area under the curve (AUC) from time 0 to infinity; Cmax = maximum concentration; CL = clearance; IR = incremental recovery based on observations; MRT0-∞ = mean residence time extrapolated toinfinity; N = number of patients; t1/2 = half-life; Vdss = volume of distribution at steady state.
Data reflects subjects in Study 984 with a minimum of five observations.- * Pharmacokinetic parameters are summarized as Mean (Standard Deviation).
Parameters* Overall
N=176 to < 12 years
N = 112 to < 18 years
N = 218 to 75 years
N = 14t1/2 [h] 54.8 (13.4) 63.1 57.4 (25.7) 53.8 (12.7) Cmax [g/L] 1.47 (0.4) 1.74 1.16 (0.5) 1.49 (0.4) AUC0-∞ [g*h/L)] 97.8 (39.1) 92.8 79.4 (52.1) 101 (40.1) MRT0-∞ [h] 80.3 (19.3) 88.6 84.8 (39.3) 79.1 (18.1) Vdss per kg [mL/kg] 62.2 (16.3) 66.8 80.8 (18.5) 59.2 (15.4) CL per kg (mL/[h*kg]) 0.836 (0.4) 0.754 1.13 (0.7) 0.801 (0.3) IR (mg/dL per mg/kg dose) 2.10 (0.6) 2.49 1.66 (0.7) 2.13 (0.6) A population pharmacokinetic model was developed that pooled the data collected in 27 patients aged 1 to 40 years who received 70 mg/kg of FESILTY. A two-compartment model was used for integrated assessments of FiAg and FiAc levels, with body weight included as a covariate to describe the pharmacokinetic data. The analysis demonstrated that the median AUC0-239h of FiAc was lower by 27.6% in patients aged < 6 years, by 13.6% in patients aged 6 to < 12 years, and by 3.4% in patients aged 12 to < 18 years compared to adult patients. The median Cmax was comparable between pediatric and adult patients.
-
14 CLINICAL STUDIES
The efficacy of FESILTY was evaluated in an open-label, single arm, multicenter, study (Study 984; NCT 02065882) in patients with congenital hypo- or afibrinogenemia. Patients with dysfibrogenemia were excluded. The study assessed FESILTY for on-demand treatment (ODT) and for on-demand prophylaxis (ODP) of bleeding events.
A total of 36 patients received FESILTY for 175 bleeding events. The median number of FESILTY infusions per bleeding event was 1 (range: 1 to 6). The mean total perioperative dose of FESILTY for 54 surgical bleeding events was 70.3 mg/kg BW for adults and 125.9 mg/kg BW for pediatric patients. The mean dose for 175 bleeding events was 70.1 mg/kg for adults and 75.8 mg/kg for pediatric patients.
The demographic characteristics of the study population were as follows: The median age was 18 years (range: 1 to 46 years) including 3 patients aged 0 to < 6 years, 9 patients aged 6 to < 12 years, and 4 patients aged 12 to < 18 years. Twenty-two patients (61%) were male and 36 patients (100%) were White. Thirty-four patients (94%) had congenital afibrinogenemia and two patients (6%) had severe hypofibrinogenemia. Out of 175 bleeding events, 45 were traumatic, 65 were spontaneous, 54 were surgical and 11 bleeds were classified as “other”. There were 60 bleeds treated with ODP and 115 bleeds with ODT.
The main efficacy endpoint was the overall hemostatic response (OHR) based on a 4-point scale assessed by the investigator as excellent, good, moderate or none. The other efficacy endpoint was the mean change in maximum clot formation (MCF) at 1 hour after infusion.
The OHR for 175 bleeding events in 36 patients was reported as excellent in 150 bleeding events (86%), good in 23 bleeding events (13%), and moderate in 2 bleeding events (1%). The mean change in MCF was 10.76 mm at 1 hour after FESILTY infusion. -
16 HOW SUPPLIED/STORAGE AND HANDLING
FESILTY is supplied in a kit containing one single-dose glass vial of human fibrinogen, one glass vial of 50 mL Sterile Water for Injection, USP, and one nextaro® v, 20/20 sterile transfer system.
Carton NDC Container NDC Fibrinogen Content 13533-502-01 13533-503-02 approximately 1 gram The actual amount of human fibrinogen in milligrams per vial is printed on the vial label and carton.
- FESILTY is not made with natural rubber latex.
- Keep FESILTY in its original carton to protect it from light.
- Store between 2oC and 30oC (36oF and 86oF). Do not freeze.
- Do not use after expiration date printed on the vial label and carton.
- Use within 4 hours after reconstitution.
- Discard unused portion.
-
17 PATIENT COUNSELING INFORMATION
Discuss following with the patient and/or caregiver:
- Hypersensitivity reactions: Inform patients and/or caregiver of the early signs of hypersensitivity reactions to FESILTY (including hives, generalized urticaria, tightness of the chest, wheezing, hypotension, and anaphylaxis), and advise them to notify their physician immediately if they experience any of these symptoms [see Warnings and Precautions (5.1)].
- Thrombosis: Inform patients and/or caregiver that blood clots with or without consequent obstruction of blood flow may occur with FESILTY. Any symptoms of blood clots such as unexplained chest and/or leg pain or swelling of the legs or arms, coughing up blood, shortness of breath, increased rate of breathing or unexplained symptoms related to the nervous system such as stroke or weakness following administration of FESILTY should be reported to their physician immediately [see Warnings and Precautions (5.2)].
- Transmission of infectious agents: Inform patients and/or caregiver that because FESILTY is made from pooled human plasma, it may carry a risk of transmitting infectious agents that can cause disease (e.g., viruses, vCJD agent, and theoretically, the CJD agent). Explain that the risk that FESILTY may transmit an infection has been reduced by screening plasma donors for prior exposure, testing donated plasma, and inactivating or removing certain viruses during manufacturing, patients should report any symptoms that concern them [see Description (11), Warnings and Precautions (5.3)].
Manufactured by:
Biotest AG
63303 Dreieich, Germany
Manufactured for:
Grifols Therapeutics LLC
Research Triangle Park, NC 27709 USA
US License No. 1871 -
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 13533-503-02 1 g Range/vial
fibrinogen,
human-chmt
FESILTY™
Lyophilized Powder 2
For intravenous use only
Rx Only
GRIFOLS
Dosage and Administration:
Read package insert.
Reconstitute with 50 mL Sterile
Water for Injection, USP.
Sterile, Non-pyrogenic
No preservative
Store between 2°C and 30°C
(36°F and 86°F). Do not freeze.
Mfd for: Grifols Therapeutics LLC,
Research Triangle Park, NC 27709 USA
US License No. 1871
Mfd by: Biotest AG, 63303 Dreieich,
Germany
(01)003 13533 503024
LOT:
EXP:
Fibrinogen
mg/vial:
Fesilty™ 1 g Range/vial
NDC: 13533-503-02
LOT:
186 034 001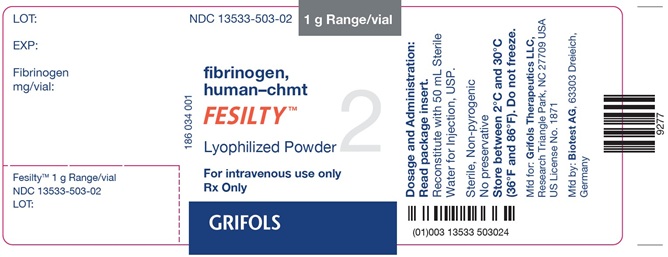
NDC: 13533-200-50 Rx Only
Sterile Water for
Injection, USP
For reconstitution of
accompanying product
Single-Dose Container. 1
GRIFOLS 50 mL
Nonpyrogenic. Do not use unless
clear. No antimicrobial agent or
other substance has been added.
Do not use for intravascular injection
without making approximately
isotonic by addition of suitable
solute. Discard unused portion.
Mfd by: Laboratorios Grifols S. A., Parets del Vallès,
Barcelona, 08150 Spain
LOT:
EXP:
186 036 001
(01)003 13533 200503
NOTE: These are shortened instructions. Full instructions
in the package insert must be read before use!
NDC: 13533-502-01 1 g Range/vial
fibrinogen human-chmt
FESILTY™
Lyophilized Powder
For intravenous use only Rx Only
GRIFOLS
This product is prepared from
large pools of human plasma
which may carry the risk of
transmitting infectious agents.
See package insert Warnings.
Sterile, Non-pyrogenic
No Preservative
If the carton seal is broken or
shows any sign of tampering, do
not use the product and notify
Grifols Therapeutics LLC
immediately.
Manufactured for:
Grifols Therapeutics LLC
Research Triangle Park, NC 27709 USA
US License No. 1871
Manufactured by: Biotest AG
63303 Dreieich, Germany
(01)003 13533 502010
GRIFOLS
183 049 001
When reconstituted with 50 mL
Sterile Water for Injection, USP,
each vial contains
approximately:
Human fibrinogen 20 mg
Arginine hydrochloride 8.43 mg
Polysorbate 80 0.51 mg
Sodium chloride 5.84 mg
Sodium citrate dihydrate 1.47 mg
Trehalose dihydrate 11.35 mg
Dosage, Reconstitution, and
Administration:
Read enclosed package insert.
Store between 2°C and 30°C
(36°F and 86°F).
Do not freeze. Keep the vial in the
carton to protect from light. Discard
unused portion.
GRIFOLS
NDC: 13533-502-01
1 g Range/vial
fibrinogen, human-chmt
FESILTY™
Lyophilized Powder
Contents:- 1 single-dose vial of Fesilty™
- 1 vial of 50 mL Sterile Water for Injection, USP
- 1 sterile nextaro® v, 20/20 filter transfer system
GRIFOLS
FESILTY™ 1 g Range/vial NDC: 13553-502-01LOT: XXXXXXXXXX
EXP: DDMMMYYYY
GTIN: 00313533502010
SN: XXXXXXXXXXXXXXXX
Fibrinogen mg/vial: XXXX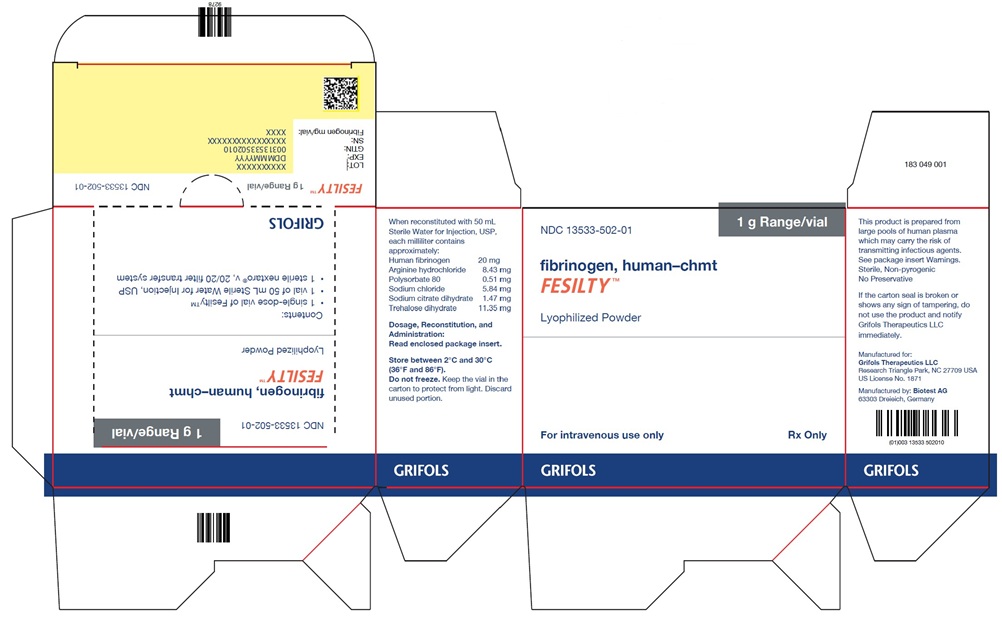
-
INGREDIENTS AND APPEARANCE
FESILTY
fibrinogen, human-chmt kitProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 13533-502 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13533-502-01 1 in 1 CARTON; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 0 VIAL, GLASS 1 mL Part 2 1 VIAL, GLASS 50 mL Part 1 of 2 FESILTY
fibrinogen, human-chmt injection, powder, lyophilized, for solutionProduct Information Item Code (Source) NDC: 13533-503 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Fibrinogen Human (UNII: N94833051K) (Fibrinogen Human - UNII:N94833051K) Fibrinogen Human 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength Arginine Hydrochloride (UNII: F7LTH1E20Y) Sodium Chloride (UNII: 451W47IQ8X) Trisodium Citrate Dihydrate (UNII: B22547B95K) Polysorbate 80 (UNII: 6OZP39ZG8H) Trehalose Dihydrate (UNII: 7YIN7J07X4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13533-503-02 50 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125833 12/16/2025 Part 2 of 2 STERILE WATER
water injectionProduct Information Item Code (Source) NDC: 13533-200 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13533-200-50 50 mL in 1 VIAL, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125833 12/16/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125833 12/16/2025 Labeler - Grifols USA LLC (048987452) Establishment Name Address ID/FEI Business Operations Biotest AG 315031799 manufacture(13533-502, 13533-503) , pack(13533-200) , label(13533-200) Establishment Name Address ID/FEI Business Operations Grifols Therapeutics LLC 611019113 manufacture(13533-503) Establishment Name Address ID/FEI Business Operations Laboratorios Grifols, S.A. 463719725 manufacture(13533-200) Establishment Name Address ID/FEI Business Operations Laboratorios Grifols, S.A. 461842294 analysis(13533-200) Establishment Name Address ID/FEI Business Operations Instituto Grifols, S.A. 465562213 analysis(13533-503) Establishment Name Address ID/FEI Business Operations Prothya Biosolutions Belgium 375250156 manufacture(13533-503) Establishment Name Address ID/FEI Business Operations TechPharm GmbH 537474223 analysis(13533-503) Establishment Name Address ID/FEI Business Operations GBA Pharma GmbH 342374604 analysis(13533-503)
Trademark Results [FESILTY]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 FESILTY 98468013 not registered Live/Pending |
GRIFOLS WORLDWIDE OPERATIONS LIMITED 2024-03-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.