DERAMSILKRX ANODYNEXA PAK- diclofenac sodium delayed release tablets, ranitidine tablets, capsaicin cream kit
DeramsilkRx Anodynexa Pak by
Drug Labeling and Warnings
DeramsilkRx Anodynexa Pak by is a Prescription medication manufactured, distributed, or labeled by Patchwerx Labs, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
Diclofenac Sodium Delayed-Release Tablets USP
Rx only
Prescribing InformationCardiovascular Risk
- NSAIDs may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk. (see WARNINGS.)
- Diclofenac sodium delayed-release tablets is contraindicated for the treatment of perioperative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS).
Gastrointestinal Risk
- NSAIDs cause an increased risk of serious gastrointestinal adverse events including inflammation, bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events. (see WARNINGS.)
DESCRIPTION
Diclofenac, as the sodium salt, is a benzene-acetic acid derivative. The chemical name is 2-[(2,6-dichlorophenyl)amino] benzeneacetic acid, monosodium salt. The molecular weight is 318.14. (Its molecular formula is C 14H 10Cl 2NaO 2, and it has the following structural formula:
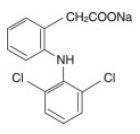
Each enteric-coated tablet for oral administration contains 75 mg of diclofenac sodium. In addition, each tablet contains the following inactive ingredients: aluminum hydrate, colloidal silicon dioxide, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, polyvinyl acetate phthalate, propylene glycol, silica, sodium alginate, sodium starch glycolate (Type A), stearic acid, synthetic black iron oxide, talc, and titanium dioxide.
CLINICAL PHARMACOLOGY
Pharmacodynamics
Diclofenac sodium delayed-release tablets, USP are a non-steroidal anti-inflammatory drug (NSAID) that exhibits anti-inflammatory, analgesic, and antipyretic activities in animal models. The mechanism of action of diclofenac sodium delayed-release, like that of other NSAIDs, is not completely understood but may be related to prostaglandin synthetase inhibition.
Pharmacokinetics
Absorption
Diclofenac is 100% absorbed after oral administration compared to IV administration as measured by urine recovery. However, due to first-pass metabolism, only about 50% of the absorbed dose is systemically available (see Table 1). Food has no significant effect on the extent of diclofenac absorption. However, there is usually a delay in the onset of absorption of 1 to 4.5 hours and a reduction in peak plasma levels of <20%.
Table 1. Pharmacokinetic Parameters for Diclofenac PK Parameter Normal Healthy Adults (18-48 yrs.) Mean Coefficient of
Variation (%)Absolute
Bioavailability (%)
[N = 7]
55
40Tmax (hr)
[N = 12]
5.3
28Oral Clearance (CL/F; mL/min)
[N = 12]
895
56Renal Clearance
(% unchanged drug in urine)
[N = 7]
<1
--Apparent Volume of
Distribution (V/F; L/kg)
[N = 56]
1.4
58Terminal Half-life (hr)
[N = 56]
2.3
48Distribution
The apparent volume of distribution (V/F) of diclofenac sodium is 1.4 L/kg.
Diclofenac is more than 99% bound to human serum proteins, primarily to albumin. Serum protein binding is constant over the concentration range (0.15-105 g/mL) achieved with recommended doses.
Diclofenac diffuses into and out of the synovial fluid. Diffusion into the joint occurs when plasma levels are higher than those in the synovial fluid, after which the process reverses and synovial fluid levels are higher than plasma levels. It is not known whether diffusion into the joint plays a role in the effectiveness of diclofenac.
Metabolism
Five diclofenac metabolites have been identified in human plasma and urine. The metabolites include 4'-hydroxy-, 5-hydroxy-, 3'-hydroxy-, 4',5-dihydroxy- and 3'-hydroxy-4'-methoxy diclofenac. The major diclofenac metabolite, 4'-hydroxy-diclofenac, has very weak pharmacologic activity. The formation of 4’-hydroxy- diclofenac is primarily mediated by CPY2C9. Both diclofenac and its oxidative metabolites undergo glucuronidation or sulfation followed by biliary excretion. Acylglucuronidation mediated by UGT2B7 and oxidation mediated by CPY2C8 may also play a role in diclofenac metabolism. CYP3A4 is responsible for the formation of minor metabolites, 5-hydroxy- and 3’-hydroxy-diclofenac. In patients with renal dysfunction, peak concentrations of metabolites 4'-hydroxy- and 5-hydroxy-diclofenac were approximately 50% and 4% of the parent compound after single oral dosing compared to 27% and 1% in normal healthy subjects.
Excretion
Diclofenac is eliminated through metabolism and subsequent urinary and biliary excretion of the glucuronide and the sulfate conjugates of the metabolites. Little or no free unchanged diclofenac is excreted in the urine. Approximately 65% of the dose is excreted in the urine and approximately 35% in the bile as conjugates of unchanged diclofenac plus metabolites. Because renal elimination is not a significant pathway of elimination for unchanged diclofenac, dosing adjustment in patients with mild to moderate renal dysfunction is not necessary. The terminal half-life of unchanged diclofenac is approximately 2 hours.
Drug Interactions
When co-administered with voriconazole (inhibitor of CYP2C9, 2C19 and 3A4 enzyme), the Cmax and AUC of diclofenac increased by 114% and 78%, respectively (see PRECAUTIONS, Drug Interactions).
Special Populations
Pediatric:The pharmacokinetics of diclofenac has not been investigated in pediatric patients.Race: Pharmacokinetic differences due to race have not been identified.
Hepatic Insufficiency:Hepatic metabolism accounts for almost 100% of diclofenac elimination, so patients with hepatic disease may require reduced doses of diclofenac compared to patients with normal hepatic function.
Renal Insufficiency: Diclofenac pharmacokinetics has been investigated in subjects with renal insufficiency. No differences in the pharmacokinetics of diclofenac have been detected in studies of patients with renal impairment. In patients with renal impairment (inulin clearance 60-90, 30-60, and <30 mL/min; N=6 in each group), AUC values and elimination rate were comparable to those in healthy subjects.
INDICATIONS AND USAGE
Carefully consider the potential bene ts and risks of diclofenac sodium delayed-release tablets and other treatment options before deciding to use diclofenac. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS).
Diclofenac is indicated:
- For relief of the signs and symptoms of osteoarthritis
- For relief of the signs and symptoms of rheumatoid arthritis
- For acute or long-term use in the relief of signs and symptoms of ankylosing spondylitis
CONTRAINDICATIONS
Diclofenac sodium delayed-release tablets is contraindicated in patients with known hypersensitivity to diclofenac.
Diclofenac should not be given to patients who have experienced asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs. Severe, rarely fatal, anaphylactic-like reactions to NSAIDs have been reported in such patients (see WARNINGS, ANAPHYLACTOID REACTIONS, and PRECAUTIONS, PREEXISTING ASTHMA).
Diclofenac is contraindicated for the treatment of perioperative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS).
WARNINGS
Cardiovascular Effects
Cardiovascular Thrombotic EventsClinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, myocar-dial infarction, and stroke, which can be fatal. All NSAIDs, both COX-2 selective and nonselective, may have a similar risk. Patients with known CV disease or risk factors for CV disease may be at greater risk. To minimize the potential risk for an adverse CV event in patients treated with an NSAID, the lowest effective dose should be used for the shortest duration possible. Physicians and patients should remain alert for the development of such events, even in the absence of previous CV symptoms. Patients should be informed about the signs and/or symptoms of serious CV events and the steps to take if they occur.
There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID does increase the risk of serious GI events (see GI WARNINGS, GI EFFECTS).
Two large, controlled, clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10-14 days following CABG surgery found an increased incidence of myocardial infarction and stroke (see CONTRAINDICATIONS).
Hypertension
NSAIDs, can lead to onset of new hypertension or worsening of preexisting hypertension, either of which may contribute to the increased incidence of CV events. Patients taking thiazides or loop diuretics may have impaired response to these therapies when taking NSAIDs. NSAIDs, including diclofenac sodium delayed-release tablets, should be used with caution in patients with hypertension. Blood pressure (BP) should be monitored closely during the initiation of NSAID treatment and throughout the course of therapy.
Congestive Heart Failure and Edema Renal Effects
Fluid retention and edema have been observed in some patients taking NSAIDs. Diclofenac should be used with caution in patients with fluid retention or heart failure.
Gastrointestinal (GI) Effects: Risk of GI Ulceration, Bleeding, and Perforation
NSAIDs, including diclofenac, can cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients, who develop a serious upper GI adverse event on NSAID therapy, is symptomatic. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occur in approximately 1% of patients treated for 3–6 months, and in about 2%–4% of patients treated for one year. These trends continue with longer duration of use, increasing the likelihood of developing a serious GI event at some time during the course of therapy. However, even short-term therapy is not without risk.
NSAIDs should be prescribed with extreme caution in those with a prior history of ulcer disease or gastrointestinal bleeding. Patients with a prior history of peptic ulcer disease and/or gastrointestinal bleeding who use NSAIDs have a greater than 10-fold increased risk for developing a GI bleed compared to patients with neither of these risk factors. Other factors that increase the risk for GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids or anticoagulants, longer duration of NSAID therapy, smoking, use of alcohol, older age, and poor general health status. Most spontaneous reports of fatal GI events are in elderly or debilitated patients and therefore, special care should be taken in treating this population.
To minimize the potential risk for an adverse GI event in patients treated with an NSAID, the lowest effective dose should be used for the shortest possible duration. Patients and physicians should remain alert for signs and symptoms of GI ulceration and bleeding during NSAID therapy and promptly initiate additional evaluation and treatment if a serious GI adverse event is suspected. This should include discontinuation of the NSAID until a serious GI adverse event is ruled out. For high risk patients, alternate therapies that do not involve NSAIDs should be considered.
Renal Effects
Caution should be used when initiating treatment with diclofenac in patients with considerable dehydration.Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of a nonsteroidal anti-inflammatory drug may cause a dose-dependent reduction in prostaglandin formation and, secondarily, in renal blood ow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics and ACE inhibitors, and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state.
Advanced Renal Disease
No information is available from controlled clinical studies regarding the use of diclofenac in patients with advanced renal disease. Therefore, treatment with diclofenac is not recommended in these patients with advanced renal disease. If diclofenac therapy must be initiated, close monitoring of the patient's renal function is advisable.Hepatic Effects
Elevations of one or more liver tests may occur during therapy with diclofenac. These laboratory abnormalities may progress, may remain unchanged, or may be transient with continued therapy. Borderline elevations (i.e., less than 3 times the ULN [ULN = the upper limit of the normal range]) or greater elevations of transaminases occurred in about 15% of diclofenac-treated patients. Of the markers of hepatic function, ALT (SGPT) is recommended for the monitoring of liver injury.In clinical trials, meaningful elevations (i.e., more than 3 times the ULN) of AST (GOT) (ALT was not measured in all studies) occurred in about 2% of approximately 5,700 patients at some time during diclofenac treatment. In a large, open-label, controlled trial of 3,700 patients treated for 2-6 months, patients were monitored first at 8 weeks and 1,200 patients were monitored again at 24 weeks. Meaningful elevations of ALT and/or AST occurred in about 4% of patients and included marked elevations (i.e., more than 8 times the ULN) in about 1% of the 3,700 patients. In that open-label study, a higher incidence of borderline (less than 3 times the ULN), moderate (3-8 times the ULN), and marked (>8 times the ULN) elevations of ALT or AST was observed in patients receiving diclofenac when compared to other NSAIDs. Elevations in transaminases were seen more frequently in patients with osteoarthritis than in those with rheumatoid arthritis.
Almost all meaningful elevations in transaminases were detected before patients became symptomatic. Abnormal tests occurred during the first 2 months of therapy with diclofenac in 42 of the 51 patients in all trials who developed marked transaminase elevations.
In postmarketing reports, cases of drug-induced hepatotoxicity have been reported in the first month, and in some cases, the first 2 months of therapy, but can occur at any time during treatment with diclofenac. Postmarketing surveillance has reported cases of severe hepatic reactions, including liver necrosis, jaundice, fulminant hepatitis with and without jaundice, and liver failure. Some of these reported cases resulted in fatalities or liver transplantation.
Physicians should measure transaminases periodically in patients receiving long-term therapy with diclofenac, because severe hepatotoxicity may develop without a prodrome of distinguishing symptoms. The optimum times for making the first and subsequent transaminase measurements are not known. Based on clinical trial data and postmarketing experiences, transaminases should be monitored within 4 to 8 weeks after initiating treatment with diclofenac. However, severe hepatic reactions can occur at any time during treatment with diclofenac.
If abnormal liver tests persist or worsen, if clinical signs and/or symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, abdominal pain, diarrhea, dark urine, etc.), diclofenac should be discontinued immediately.
To minimize the possibility that hepatic injury will become severe between transaminase measurements, physicians should inform patients of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, diarrhea, pruritus, jaundice, right upper quadrant tenderness, and “ u-like” symptoms), and the appropriate action patients should take if these signs and symptoms appear.
To minimize the potential risk for an adverse liver related event in patients treated with diclofenac, the lowest effective dose should be used for the shortest duration possible. Caution should be exercised in prescribing diclofenac with concomitant drugs that are known to be potentially hepatotoxic (e.g., antibiotics, antiepileptics).
Anaphylactoid Reactions
As with other NSAIDs, anaphylactoid reactions may occur in patients without known prior exposure to diclofenac. Diclofenac should not be given to patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs. (See CONTRAINDICATIONS and PRECAUTIONS, PREEXISTING ASTHMA.) Emergency help should be sought in cases where an anaphylactoid reaction occurs.Skin Reactions
NSAIDs, including diclofenac, can cause serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Patients should be informed about the signs and symptoms of serious skin manifestations and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity.Pregnancy
In late pregnancy, as with other NSAIDs, diclofenac should be avoided because it may cause premature closure of the ductus arteriosus.PRECAUTIONS
General
Diclofenac sodium delayed-release tablets cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease exacerbation. Patients on prolonged corticosteroid therapy should have their therapy tapered slowly if a decision is made to discontinue corticosteroids.The pharmacological activity of diclofenac in reducing fever and inflammation may diminish the utility of these diagnostic signs in detecting complications of presumed noninfectious, painful conditions.
Hematological Effects
Anemia is sometimes seen in patients receiving NSAIDs, including diclofenac. This may be due to fluid retention, occult or gross GI blood loss, or an incompletely described effect upon erythropoiesis. Patients on long-term treatment with NSAIDs, including diclofenac, should have their hemoglobin or hematocrit checked if they exhibit any signs or symptoms of anemia.NSAIDs inhibit platelet aggregation and have been shown to prolong bleeding time in some patients. Unlike aspirin, their effect on platelet function is quantitatively less, of shorter duration, and reversible. Patients receiving diclofenac who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants, should be carefully monitored.
Preexisting Asthma
Patients with asthma may have aspirin-sensitive asthma. The use of aspirin in patients with aspirin-sensitive asthma has been associated with severe bronchospasm which can be fatal. Since cross-reactivity, including bronchospasm, between aspirin and other nonsteroidal anti-inflammatory drugs has been reported in such aspirin-sensitive patients, diclofenac should not be administered to patients with this form of aspirin sensitivity and should be used with caution in patients with preexisting asthma.Information for Patients
Patients should be informed of the following information before initiating therapy with an NSAID and periodically during the course of ongoing therapy. Patients should also be encouraged to read the NSAID Medication Guide that accompanies each prescription dispensed.- Diclofenac, like other NSAIDs, may cause serious CV side effects, such as MI or stroke, which may result in hospitalization and even death. Although serious CV events can occur without warning symptoms, patients should be alert for the signs and symptoms of chest pain, shortness of breath, weakness, slurring of speech, and should ask for medical advice when observing any indicative sign or symptoms. Patients should be apprised of the importance of this follow-up (see WARNINGS, CARDIOVASCULAR EFFECTS).
- Diclofenac, like other NSAIDs, can cause GI discomfort and, rarely, more serious GI side effects, such as ulcers and bleeding, which may result in hospitalization and even death. Although serious GI tract ulcerations and bleeding can occur without warning symptoms, patients should be alert for the signs and symptoms of ulcerations and bleeding, and should ask for medical advice when observing any indicative sign or symptoms including epigastric pain, dyspepsia, melena, and hematemesis. Patients should be apprised of the importance of this follow-up (see WARNINGS, GASTROINTESTINAL EFFECTS: RISK OF ULCERATION, BLEEDING, AND PERFORATION).
- Diclofenac, like other NSAIDs, can cause serious skin side effects such as exfoliative dermatitis, SJS, and TEN, which may result in hospitalizations and even death. Although serious skin reactions may occur without warning, patients should be alert for the signs and symptoms of skin rash and blisters, fever, or other signs of hypersensitivity such as itching, and should ask for medical advice when observing any indicative signs or symptoms. Patients should be advised to stop the drug immediately if they develop any type of rash and contact their physicians as soon as possible.
- Patients should promptly report signs or symptoms of unexplained weight gain or edema to their physicians.
- Patients should be informed of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, jaundice, right upper quadrant tenderness, and “ u-like” symptoms). If these occur, patients should be instructed to stop therapy and seek immediate medical therapy (see WARNINGS, HEPATIC EFFECTS).
- Patients should be informed of the signs of an anaphylactoid reaction (e.g., difficulty breathing, swelling of the face or throat). If these occur, patients should be instructed to seek immediate emergency help (see WARNINGS).
- In late pregnancy, as with other NSAIDs, diclofenac should be avoided because it will cause premature closure of the ductus arteriosus.
Laboratory Tests
Because serious GI tract ulcerations and bleeding can occur without warning symptoms, physicians should monitor for signs or symptoms of GI bleeding. In patients on long-term treatment with NSAIDs, including diclofenac, the CBC and a chemistry profile (including transaminase levels) should be checked periodically. If clinical signs and symptoms consistent with liver or renal disease develop, systemic manifestations occur (e.g., eosinophilia, rash, etc.) or if abnormal liver tests persist or worsen, diclofenac should be discontinued.Drug Interactions
Aspirin: When diclofenac is administered with aspirin, its protein binding is reduced. The clinical significance of this interaction is not known; however, as with other NSAIDs, concomitant administration of diclofenac and aspirin is not generally recommended because of the potential of increased adverse effects.Methotrexate:NSAIDs have been reported to competitively inhibit methotrexate accumulation in rabbit kidney slices. This may indicate that they could enhance the toxicity of methotrexate. Caution should be used when NSAIDs are administered concomitantly with methotrexate.
Cyclosporine: Diclofenac, like other NSAIDs, may affect renal prostaglandins and increase the toxicity of certain drugs. Therefore, concomitant therapy with diclofenac may increase cyclosporine's nephrotoxicity. Caution should be used when diclofenac is administered concomitantly with cyclosporine.
ACE Inhibitors: Reports suggest that NSAIDs may diminish the antihypertensive effect of ACE inhibitors. This interaction should be given consideration in patients taking NSAIDs concomitantly with ACE inhibitors.
Furosemide: Clinical studies, as well as postmarketing observations, have shown that diclofenac can reduce the natriuretic effect of furosemide and thiazides in some patients. This response has been attributed to inhibition of renal prostaglandin synthesis. During concomitant therapy with NSAIDs, the patient should be observed closely for signs of renal failure (see WARNINGS, RENAL EFFECTS), as well as to assure diuretic efficacy.
Lithium: NSAIDs have produced an elevation of plasma lithium levels and a reduction in renal lithium clearance. The mean minimum lithium concentration increased 15% and the renal clearance was decreased by approximately 20%. These effects have been attributed to inhibition of renal prostaglandin synthesis by the NSAID. Thus, when NSAIDs and lithium are administered concurrently, subjects should be observed carefully for signs of lithium toxicity.
Warfarin: The effects of warfarin and NSAIDs on GI bleeding are synergistic, such that users of both drugs together have a risk of serious GI bleeding higher than users of either drug alone.
CYP2C9 Inhibitors or Inducers: Diclofenac is metabolized by cytochrome P450 enzymes, predominantly by CYP2C9. Co-administration of diclofenac with CYP2C9 inhibitors (e.g. voriconazole) may enhance the exposure and toxicity of diclofenac whereas co-administration with CYP2C9 inducers (e.g. rifampin) may lead to compromised efficacy of diclofenac. Use caution when dosing diclofenac with CYP2C9 inhibitors or inducers; a dosage adjustment may be warranted (see CLINICAL PHARMACOLOGY, Pharmacokinetics, Drug Interactions).
Pregnancy
Teratogenic Effects: Pregnancy Category CReproductive studies conducted in rats and rabbits have not demonstrated evidence of developmental abnormalities. However, animal reproduction studies are not always predictive of human response. There are no adequate and well-controlled studies in pregnant women.
Nonteratogenic Effects: Because of the known effects of nonsteroidal anti-inflammatory drugs on the fetal cardiovascular system (closure of ductus arteriosus), use during pregnancy (particularly late pregnancy) should be avoided.
Labor and Delivery
In rat studies with NSAIDs, as with other drugs known to inhibit prostaglandin synthesis, an increased incidence of dystocia, delayed parturition, and decreased pup survival occurred. The effects of diclofenac on labor and delivery in pregnant women are unknown.Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from diclofenac, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.Pediatric Use
Safety and effectiveness in pediatric patients have not been established.Geriatric Use
As with any NSAIDs, caution should be exercised in treating the elderly (65 years and older).ADVERSE REACTIONS
In patients taking diclofenac sodium delayed-release tablets, or other NSAIDs, the most frequently reported adverse experiences occurring in approximately 1%–10% of patients are:
- Gastrointestinal experiences including: abdominal pain, constipation, diarrhea, dyspepsia, atulence, gross bleeding/ perforation, heartburn, nausea, GI ulcers (gastric/duodenal) and vomiting.
- Abnormal renal function, anemia, dizziness, edema, elevated liver enzymes, headaches, increased bleeding time, pruritus, rashes and tinnitus.
Additional adverse experiences reported occasionally include:
Body as a Whole:fever, infection, sepsis
Cardiovascular System: congestive heart failure, hypertension, tachycardia, syncope
Digestive System: dry mouth, esophagitis, gastric/peptic ulcers, gastritis, gastrointestinal bleeding, glossitis, hematemesis, hepatitis, jaundice
Hemic and Lymphatic System: ecchymosis, eosinophilia, leukopenia, melena, purpura, rectal bleeding, stomatitis, thrombocytopenia
Metabolic and Nutritional: weight changes
Nervous System: anxiety, asthenia, confusion, depression, dream abnormalities, drowsiness, insomnia, malaise, nervousness, paresthesia, somnolence, tremors, vertigo
Respiratory System: asthma, dyspnea
Skin and Appendages: alopecia, photosensitivity, sweating increased
Special Senses: blurred vision
Urogenital System: cystitis, dysuria, hematuria, interstitial nephritis, oliguria/polyuria, proteinuria, renal failure
Other adverse reactions, which occur rarely are:
Body as a Whole: anaphylactic reactions, appetite changes, death
Cardiovascular System: arrhythmia, hypotension, myocardial infarction, palpitations, vasculitis
Digestive System:colitis, eructation, liver failure, pancreatitis
Hemic and Lymphatic System:agranulocytosis, hemolytic anemia, aplastic anemia, lymphadenopathy, pancytopenia
Metabolic and Nutritional: hyperglycemia
Nervous System: convulsions, coma, hallucinations, meningitis
Respiratory System: respiratory depression, pneumonia
Skin and Appendages: angioedema, toxic epidermal necrolysis, erythema multiforme, exfoliative dermatitis, Stevens-Johnson syndrome, urticaria
Special Senses: conjunctivitis, hearing impairment
OVERDOSAGE
Symptoms following acute NSAID overdoses are usually limited to lethargy, drowsiness, nausea, vomiting, and epigastric pain, which are generally reversible with supportive care. Gastrointestinal bleeding can occur. Hypertension, acute renal failure, respiratory depression and coma may occur, but are rare. Anaphylactoid reactions have been reported with therapeutic ingestion of NSAIDs, and may occur following an overdose.
Patients should be managed by symptomatic and supportive care following a NSAID overdose. There are no specific antidotes. Emesis and/or activated charcoal (60 to 100 g in adults, 1 to 2 g/kg in children) and/or osmotic cathartic may be indicated in patients seen within 4 hours of ingestion with symptoms or following a large overdose (5 to 10 times the usual dose). Forced diuresis, alkalinization of urine, hemodialysis, or hemoperfusion may not be useful due to high protein binding.
DOSAGE AND ADMINISTRATION
Carefully consider the potential benefits and risks of diclofenac sodium delayed-release tablets and other treatment options before deciding to use diclofenac. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS).
After observing the response to initial therapy with diclofenac, the dose and frequency should be adjusted to suit an individual patient's needs.
For the relief of osteoarthritis, the recommended dosage is 100 to 150 mg/day in divided doses (50 mg b.i.d. or t.i.d., or 75 mg b.i.d.).
For the relief of rheumatoid arthritis, the recommended dosage is 150 to 200 mg/day in divided doses (50 mg t.i.d. or q.i.d., or 75 mg b.i.d.).
For the relief of ankylosing spondylitis, the recommended dosage is 100 to 125 mg/day, administered as 25 mg q.i.d., with an extra 25-mg dose at bedtime if necessary.
Different formulations of diclofenac (diclofenac sodium delayed-release tablets; diclofenac sodium extended-release tablets; diclofenac potassium immediate-release tablets) are not necessarily bioequivalent even if the milligram strength is the same.
HOW SUPPLIED
Diclofenac Sodium Delayed-Release Tablets, USP are available as follows:
75 mg — Each white, round, enteric-coated tablet printed with R on one side and 551 on the other side with black ink contains 75 mg of Diclofenac Sodium, USP. Tablets are supplied in bottles of 60 (NDC: 0228-2551-06).
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F).
Protect from moisture.
Dispense in a tight, light-resistant container as defined in the USP.MEDICATION GUIDE
Medication Guide for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
(See the end of this Medication Guide for a list of prescription NSAID medicines.)
What is the most important information I should know about medicines called Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?
NSAID medicines may increase the chance of a heart attack or stroke that can lead to death.
This chance increases:
- with longer use of NSAID medicines
- in people who have heart disease
NSAID medicines should never be used right before or after a heart surgery called a coronary artery bypass graft (CABG).
NSAID medicines can cause ulcers and bleeding in the stomach and intestines at any time during treatment. Ulcers and bleeding:
- can happen without warning symptoms
- may cause death
The chance of a person getting an ulcer or bleeding increases with:
- taking medicines called corticosteroids and anticoagulants
- longer use
- smoking
- drinking alcohol
- older age
- having poor health
NSAID medicines should only be used:
- exactly as prescribed
- at the lowest dose possible for your treatment
- for the shortest time needed
What are Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?
NSAID medicines are used to treat pain and redness, swelling, and heat (inflammation) from medical conditions such as:
- different types of arthritis
- menstrual cramps and other types of short-term pain
Who should not take a Non-Steroidal Anti-Inflammatory Drug (NSAID)?
Do not take an NSAID medicine:
- if you had an asthma attack, hives, or other allergic reaction with aspirin or any other NSAID medicine
- for pain right before or after heart bypass surgery
Tell your healthcare provider:
- about all your medical conditions.
- about all of the medicines you take. NSAIDs and some other medicines can interact with each other and cause serious side effects.
Keep a list of your medicines to show to your healthcare provider and pharmacist.
- if you are pregnant. NSAID medicines should not be used by pregnant women late in their pregnancy.
- if you are breastfeeding. Talk to your doctor.
What are the possible side effects of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?
Serious side effects include: - heart attack
- stroke
- high blood pressure
- heart failure from body swelling (fluid retention)
- kidney problems including kidney failure
- bleeding and ulcers in the stomach and intestine
- low red blood cells (anemia)
- life-threatening skin reactions
- life-threatening allergic reactions
- liver problems including liver failure
- asthma attacks in people who have asthma
Other side effects include: - heartburn
- nausea
- vomiting
- dizziness
- stomach pain
- constipation
- diarrhea
- gas
Get emergency help right away if you have any of the following symptoms:
- shortness of breath or trouble breathing
- chest pain
- weakness in one part or side of your body
- slurred speechswelling of the face or throat
Stop your NSAID medicine and call your healthcare provider right away if you have any of the following symptoms:
- nausea
- more tired or weaker than usual
- itching
- your skin or eyes look yellow
- stomach pain
- flu-like symptoms
- vomit blood
- there is blood in your bowel movement or it is black and sticky like tar
- skin rash or blisters with fever
- unusual weight gain
- swelling of the arms and legs, hands and feet
These are not all the side effects with NSAID medicines. Talk to your healthcare provider or pharmacist for more information about NSAID medicines. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Other information about Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
- Aspirin is an NSAID medicine but it does not increase the chance of a heart attack. Aspirin can cause bleeding in the brain, stomach, and intestines. Aspirin can also cause ulcers in the stomach and intestines.
- Some of these NSAID medicines are sold in lower doses without a prescription (over the counter). Talk to your healthcare provider before using over the counter NSAIDs for more than 10 days.
NSAID Medicines That Need A Prescription
Generic Name Tradename Celecoxib Celebrex Diclofenac Cataflam, Voltaren,
Arthrotec (combined with
misoprostol)
Diflunisal Dolobid Etodolac Lodine, Lodine XL Fenoprofen Nalfon, Nalfon 200 Flurbiprofen Ansaid Ibuprofen Motrin, Tab-Profen,
Vicoprofen* (combined with
hydrocodone), Combunox
(combined with oxycodone)Indomethacin Indocin, Indocin SR,
Indo-Lemmon,
IndomethaganKetoprofen Oruvail Ketorolac Toradol Mefenamic Acid Ponstel Meloxicam Mobic Nabumetone Relafen Naproxen Naprosyn, Anaprox,
Anaprox DS, EC-Naproxyn,
Naprelan, Naprapac
(copackaged with
lansoprazole)Oxaprozin Daypro Piroxicam Feldene Sulindac Clinoril Tolmetin Tolectin, Tolectin DS,
Tolectin 600* Vicoprofen contains the same dose of ibuprofen as over-the-counter (OTC) NSAIDs, and is usually used for less than 10 days to treat pain. The OTC NSAID label warns that long term continuous use may increase the risk of heart attack or stroke.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
-
Ranitidine Tablets USP
Rx only
DESCRIPTION
The active ingredient in Ranitidine Tablets, USP 150 mg is ranitidine hydrochloride (HCl), USP, a histamine H 2-receptor antagonist. Chemically it is N[2-[[[5-[(dimethylamino)methyl]-2-furanyl]methyl]thio]ethyl]-N'-methyl-2-nitro-1,1-ethenediamine, HCl. It has the following structure:
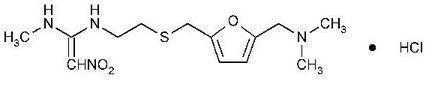
The empirical formula is C 13H 22N 4O 3S·HCl, representing a molecular weight of 350.87. Ranitidine HCl is a white to pale yellow, granular substance that is soluble in water. It has a slightly bitter taste and sulfurlike odor.
Each Ranitidine Tablets, USP 150 mg for oral administration contains 167.4 mg of ranitidine HCl equivalent to 150 mg of ranitidine. Each tablet also contains the inactive ingredients colloidal silicon dioxide, croscarmellose sodium, hypromellose, magnesium stearate, microcrystalline cellulose, polydextrose, titanium dioxide, triethyl citrate and FD&C Yellow #6.
CLINICAL PHARMACOLOGY
Ranitidine Tablets, USP are a competitive, reversible inhibitor of the action of histamine at the histamine H 2-receptors, including receptors on the gastric cells. Ranitidine Tablets, USP do not lower serum Ca++ in hypercalcemic states. Ranitidine Tablets, USP are not an anticholinergic agent.
Pharmacokinetics:
Absorption: Ranitidine Tablets, USP are 50% absorbed after oral administration, compared to an intravenous (IV) injection with mean peak levels of 440 to 545 ng/mL occurring 2 to 3 hours after a 150-mg dose. Absorption is not significantly impaired by the administration of food or antacids. Propantheline slightly delays and increases peak blood levels of ranitidine, probably by delaying gastric emptying and transit time. In one study, simultaneous administration of high-potency antacid (150 mmol) in fasting subjects has been reported to decrease the absorption of Ranitidine Tablets, USP.
Distribution: The volume of distribution is about 1.4 L/kg. Serum protein binding averages 15%.
Metabolism: In humans, the N-oxide is the principal metabolite in the urine; however, this amounts to <4% of the dose. Other metabolites are the S-oxide (1%) and the desmethyl ranitidine (1%). The remainder of the administered dose is found in the stool. Studies in patients with hepatic dysfunction (compensated cirrhosis) indicate that there are minor, but clinically insignificant, alterations in ranitidine half-life, distribution, clearance, and bioavailability.
Excretion: The principal route of excretion is the urine, with approximately 30% of the orally administered dose collected in the urine as unchanged drug in 24 hours. Renal clearance is about 410 mL/min, indicating active tubular excretion. The elimination half-life is 2.5 to 3 hours. Four patients with clinically significant renal function impairment (creatinine clearance 25 to 35 mL/min) administered 50 mg of ranitidine intravenously had an average plasma half-life of 4.8 hours, a ranitidine clearance of 29 mL/min, and a volume of distribution of 1.76 L/kg. In general, these parameters appear to be altered in proportion to creatinine clearance (see DOSAGE AND ADMINISTRATION).
Geriatrics: The plasma half-life is prolonged and total clearance is reduced in the elderly population due to a decrease in renal function. The elimination half-life is 3 to 4 hours. Peak levels average 526 ng/mL following a 150-mg twice daily dose and occur in about 3 hours (see PRECAUTIONS: Geriatric Use and DOSAGE AND ADMINISTRATION: Dosage Adjustment for Patients With Impaired Renal Function).
Pediatrics: There are no significant differences in the pharmacokinetic parameter values for ranitidine in pediatric patients (from 1 month up to 16 years of age) and healthy adults when correction is made for body weight. The average bioavailability of ranitidine given orally to pediatric patients is 48% which is comparable to the bioavailability of ranitidine in the adult population. All other pharmacokinetic parameter values (t1/2 , Vd, and CL) are similar to those observed with intravenous ranitidine use in pediatric patients. Estimates of Cmax and Tmax are displayed in Table 1.
Table 1. Ranitidine Pharmacokinetics in Pediatric Patients Following Oral Dosing Population (age) n Dosage Form
(dose)C max
(ng/mLT max
(hours)Gastric or duodenal ulcer
(3.5 to 16 years)12 Tablets
(1 to 2 mg/kg)54 to 492 2 Otherwise healthy requiring Ranitidine
(0.7 to 14 years, Single dose)10 Syrup
(2 mg/kg)244 1.61 Otherwise healthy requiring Ranitidine
(0.7 to 14 years, Multiple dose)10 Syrup
(2 mg/kg)320 1.66 Plasma clearance measured in 2 neonatal patients (less than 1 month of age) was considerably lower (3 mL/min/kg) than children or adults and is likely due to reduced renal function observed in this population (see PRECAUTIONS: Pediatric Use and DOSAGE AND ADMINISTRATION: Pediatric Use).
Pharmacodynamics: Serum concentrations necessary to inhibit 50% of stimulated gastric acid secretion are estimated to be 36 to 94 ng/mL. Following a single oral dose of 150 mg, serum concentrations of ranitidine are in this range up to 12 hours. However, blood levels bear no consistent relationship to dose or degree of acid inhibition.Plasma clearance measured in 2 neonatal patients (less than 1 month of age) was considerably lower (3 mL/min/kg) than children or adults and is likely due to reduced renal function observed in this population (see PRECAUTIONS: Pediatric Use and DOSAGE AND ADMINISTRATION: Pediatric Use).Antisecretory Activity:
1. Effects on Acid Secretion: Ranitidine Tablets, USP inhibit both daytime and nocturnal basal gastric acid secretions as well as gastric acid secretion stimulated by food, betazole, and pentagastrin, as shown in Table 2.
Table 2. Effect of Oral Ranitidine Tablets, USP on Gastric Acid Secretion Time After
Dose, hours% Inhibition of Gastric Acid Output by Dose, mg 75 to 80 100 150 200 Basal Up to 4 99 95 Nocturnal Up to 13 95 96 92 Betazole Up to 3 97 99 Pentagastrin Up to 5 58 72 72 80 Meal Up to 3 73 79 95 It appears that basal-, nocturnal-, and betazole-stimulated secretions are most sensitive to inhibition by Ranitidine Tablets, USP, responding almost completely to doses of 100 mg or less, while pentagastrin- and food-stimulated secretions are more difficult to suppress.
2. Effects on Other Gastrointestinal Secretions:
Pepsin: Oral Ranitidine Tablets, USP do not affect pepsin secretion. Total pepsin output is reduced in proportion to the decrease in volume of gastric juice.
Intrinsic Factor: Oral Ranitidine Tablets, USP have no significant effect on pentagastrin-stimulated intrinsic factor secretion.
Serum Gastrin: Ranitidine Tablets, USP have little or no effect on fasting or postprandial serum gastrin.
Other Pharmacologic Actions:
- Gastric bacterial flora-increase in nitrate-reducing organisms, significance not known.
- Prolactin levels-no effect in recommended oral or intravenous (IV) dosage, but small, transient, dose-related increases in serum prolactin have been reported after IV bolus injections of 100 mg or more.
- Other pituitary hormones-no effect on serum gonadotropins, TSH, or GH. Possible impairment of vasopressin release.
- No change in cortisol, aldosterone, androgen, or estrogen levels.
- No antiandrogenic action.
- No effect on count, motility, or morphology of sperm.
Pediatrics: Oral doses of 6 to 10 mg/kg/day in two or three divided doses maintain gastric pH>4 throughout most of the dosing interval.
Clinical Trials:
Active Duodenal Ulcer: In a multicenter, double-blind, controlled, US study of endoscopically diagnosed duodenal ulcers, earlier healing was seen in the patients treated with Ranitidine Tablets, USP as shown in Table 3.
Table 3. Duodenal Ulcer Patient Healing Rates Ranitidine Tablets, USP * Placebo* Number
EnteredHealed /
EvaluableNumber
EnteredHealed /
EvaluableOutpatients 195 69/182
(38%) †188 31-164
(19%)Week 2 Week 4 137/187
(73%) †76/168
(45%)*All patients were permitted antacids as needed for relief of pain.
†P<0.0001.
In these studies, patients treated with Ranitidine Tablets, USP reported a reduction in both daytime and nocturnal pain, and they also consumed less antacid than the placebo-treated patients.
Table 4. Mean Daily Doses of Antacid Ulcer Healed Ulcer Not Healed Ranitidine 0.06 0.71 Placebo 0.71 1.43 Foreign studies have shown that patients heal equally well with 150 mg twice daily and 300 mg at bedtime (85% versus 84%, respectively) during a usual 4-week course of therapy. If patients require extended therapy of 8 weeks, the healing rate may be higher for 150 mg twice daily as compared to 300 mg at bedtime (92% versus 87%, respectively).
Studies have been limited to short-term treatment of acute duodenal ulcer. Patients whose ulcers healed during therapy had recurrences of ulcers at the usual rates.
Maintenance Therapy in Duodenal Ulcer: Ranitidine has been found to be effective as maintenance therapy for patients following healing of acute duodenal ulcers. In 2 independent, double-blind, multicenter, controlled trials, the number of duodenal ulcers observed was significantly less in patients treated with Ranitidine Tablets, USP (150 mg at bedtime) than in patients treated with placebo over a 12-month period.
Table 5. Duodenal Ulcer Prevalence Double-Blind, Multicenter, Placebo-Controlled Trials Multicenter
TrialDrug Duodenal Ulcer Prevalence No. of Patients 0 to 4
Months0 to 8
Months0 to 12
MonthsUSA RAN 20%* 24%* 35%* 138 PLC 44% 54% 59% 139 Foreign RAN 12%* 21%* 28%* 174 PLC 56% 64% 68% 165 % = Life table estimate.
* = P<0.05 (Ranitidine Tablets, USP versus comparator).
RAN = ranitidine (Ranitidine Tablets, USP)
PLC = placebo.
As with other H 2-antagonists, the factors responsible for the significant reduction in the prevalence of duodenal ulcers include prevention of recurrence of ulcers, more rapid healing of ulcers that may occur during maintenance therapy, or both.
Gastric Ulcer: In a multicenter, double-blind, controlled, US study of endoscopically diagnosed gastric ulcers, earlier healing was seen in the patients treated with Ranitidine Tablets, USP as shown in Table 6.
Table 6. Gastric Ulcer Patient Healing Rates Ranitidine Tablets, USP* Placebo* Number
EnteredHealed /
EvaluableNumber
EnteredHealed /
EvaluableOutpatients 92 16/83
(19%) †94 10/83
(12%)Week 2 Week 6 50/73
(68%) †35/69
(51%)*All patients were permitted antacids as needed for relief of pain.
† P = 0.009.
In this multicenter trial, significantly more patients treated with Ranitidine Tablets, USP became pain free during therapy.
Maintenance of Healing of Gastric Ulcers: In two multicenter, double-blind, randomized, placebo-controlled, 12-month trials conducted in patients whose gastric ulcers had been previously healed, Ranitidine Tablets, USP 150 mg at bedtime was significantly more effective than placebo in maintaining healing of gastric ulcers.
Pathological Hypersecretory Conditions (such as Zollinger-Ellison syndrome):
Ranitidine Tablets, USP inhibits gastric acid secretion and reduces occurrence of diarrhea, anorexia, and pain in patients with pathological hypersecretion associated with Zollinger-Ellison syndrome, systemic mastocytosis, and other pathological hypersecretory conditions (e.g., postoperative, "short-gut" syndrome, idiopathic). Use of Ranitidine Tablets, USP was followed by healing of ulcers in 8 of 19 (42%) patients who were intractable to previous therapy.
Gastroesophageal Reflux Disease (GERD): In 2 multicenter, double-blind, placebo-controlled, 6-week trials performed in the United States and Europe, Ranitidine Tablets, USP 150 mg twice daily was more effective than placebo for the relief of heartburn and other symptoms associated with GERD. Ranitidine-treated patients consumed significantly less antacid than did placebo-treated patients.
The US trial indicated that Ranitidine Tablets, USP 150 mg twice daily significantly reduced the frequency of heartburn attacks and severity of heartburn pain within 1 to 2 weeks after starting therapy. The improvement was maintained throughout the 6-week trial period. Moreover, patient response rates demonstrated that the effect on heartburn extends through both the day and night time periods.
In two additional US multicenter, double-blind, placebo-controlled, 2-week trials, Ranitidine Tablets, USP 150 mg twice daily was shown to provide relief of heartburn pain within 24 hours of initiating therapy and a reduction in the frequency of severity of heartburn.
Erosive Esophagitis: In two multicenter, double-blind, randomized, placebo-controlled, 12-week trials performed in the United States, Ranitidine Tablets, USP 150 mg four times daily was significantly more effective than placebo in healing endoscopically diagnosed erosive esophagitis and in relieving associated heartburn.
The erosive esophagitis healing rates were as follows:
Table 7. Erosive Esophagitis Patient Healing Rates Healed / Evaluable Placebo*
n=229Ranitidine Tablets, USP
150 mg 4 times daily*
n=215Week 4 43/198 (22%) 96/206 (47%) † Week 8 63/176 (36%) 142/200 (71%) † Week 12 92/159 (58%) 162/192 (84%) † *All patients were permitted antacids as needed for relief of pain.
† P<0.001 versus placebo.
No additional benefit in healing of esophagitis or in relief of heartburn was seen with a ranitidine dose of 300 mg 4 times daily.
Maintenance of Healing of Erosive Esophagitis: In 2 multicenter, double-blind, randomized, placebo-controlled, 48-week trials conducted in patients whose erosive esophagitis had been previously healed, Ranitidine Tablets, USP 150 mg twice daily was significantly more effective than placebo in maintaining healing of erosive esophagitis.
INDICATIONS AND USAGE
Ranitidine Tablets, USP are indicated in:
- Short-term treatment of active duodenal ulcer. Most patients heal within 4 weeks. Studies available to date have not assessed the safety of ranitidine in uncomplicated duodenal ulcer for periods of more than 8 weeks.
- Maintenance therapy for duodenal ulcer patients at reduced dosage after healing of acute ulcers. No placebo-controlled comparative studies have been carried out for periods of longer than 1 year.
- The treatment of pathological hypersecretory conditions (e.g., Zollinger-Ellison syndrome and systemic mastocytosis).
- Short-term treatment of active, benign gastric ulcer. Most patients heal within 6 weeks and the usefulness of further treatment has not been demonstrated. Studies available to date have not assessed the safety of ranitidine in uncomplicated, benign gastric ulcer for periods of more than 6 weeks.
- Maintenance therapy for gastric ulcer patients at reduced dosage after healing of acute ulcers. Placebo-controlled studies have been carried out for 1 year.
- Treatment of GERD. Symptomatic relief commonly occurs within 24 hours after starting therapy with Ranitidine Tablets, USP 150 mg b.i.d.
- Treatment of endoscopically diagnosed erosive esophagitis. Symptomatic relief of heartburn commonly occurs within 24 hours of therapy initiation with Ranitidine Tablets, USP 150 mg q.i.d.
- Maintenance of healing of erosive esophagitis. Placebo-controlled trials have been carried out for 48 weeks.
Concomitant antacids should be given as needed for pain relief to patients with active duodenal ulcer; active, benign gastric ulcer; hypersecretory states; GERD; and erosive esophagitis.
CONTRAINDICATIONS
Ranitidine Tablets, USP is contraindicated for patients known to have hypersensitivity to the drug or any of the ingredients (see PRECAUTIONS).
PRECAUTIONS
General:
- Symptomatic response to therapy with Ranitidine Tablets, USP does not preclude the presence of gastric malignancy.
- Since ranitidine is excreted primarily by the kidney, dosage should be adjusted in patients with impaired renal function (see DOSAGE AND ADMINISTRATION). Caution should be observed in patients with hepatic dysfunction since ranitidine is metabolized in the liver.
- Rare reports suggest that ranitidine may precipitate acute porphyric attacks in patients with acute porphyria. Ranitidine should therefore be avoided in patients with a history of acute porphyria.
Laboratory Tests: False-positive tests for urine protein with MULTISTIX ® may occur during therapy with Ranitidine Tablets, USP, and therefore testing with sulfosalicylic acid is recommended.
Drug Interactions: Ranitidine Tablets, USP has been reported to affect the bioavailability of other drugs through several different mechanisms such as competition for renal tubular secretion, alteration of gastric pH, and inhibition of cytochrome P450 enzymes.
Procainamide: Ranitidine, a substrate of the renal organic cation transport system, may affect the clearance of other drugs eliminated by this route. High doses of ranitidine (e.g., such as those used in the treatment of Zollinger-Ellison syndrome) have been shown to reduce the renal excretion of procainamide and N-acetylprocainamide resulting in increased plasma levels of these drugs. Although this interaction is unlikely to be clinically relevant at usual ranitidine doses, it may be prudent to monitor for procainamide toxicity when administered with oral ranitidine at a dose exceeding 300 mg per day.
Warfarin:There have been reports of altered prothrombin time among patients on concomitant warfarin and ranitidine therapy. Due to the narrow therapeutic index, close monitoring of increased or decreased prothrombin time is recommended during concurrent treatment with ranitidine.
Ranitidine may alter the absorption of drugs in which gastric pH is an important determinant of bioavailability. This can result in either an increase in absorption (e.g., triazolam, midazolam, glipizide) or a decrease in absorption (e.g., ketoconazole, atazanavir, delavirdine, gefitinib). Appropriate clinical monitoring is recommended.
Atazanavir: Atazanavir absorption may be impaired based on known interactions with other agents that increase gastric pH. Use with caution. See atazanavir label for specific recommendations.
Delavirdine: Delavirdine absorption may be impaired based on known interactions with other agents that increase gastric pH. Chronic use of H2-receptor antagonists with delavirdine is not recommended.
Gefitinib: Gefitinib exposure was reduced by 44% with the co-administration of ranitidine and sodium bicarbonate (dosed to maintain gastric pH above 5.0). Use with caution.
Glipizide: In diabetic patients, glipizide exposure was increased by 34% following a single 150-mg dose of oral ranitidine. Use appropriate clinical monitoring when initiating or discontinuing ranitidine.
Ketoconazole: Oral ketoconazole exposure was reduced by up to 95% when oral ranitidine was co-administered in a regimen to maintain a gastric pH of 6 or above. The degree of interaction with usual dose of ranitidine (150 mg twice daily) is unknown.
Midazolam: Oral midazolam exposure in 5 healthy volunteers was increased by up to 65% when administered with oral ranitidine at a dose of 150 mg twice daily. However, in another interaction study in 8 volunteers receiving IV midazolam, a 300 mg oral dose of ranitidine increased midazolam exposure by about 9%. Monitor patients for excessive or prolonged sedation when ranitidine is co-administered with oral midazolam.
Triazolam: Triazolam exposure in healthy volunteers was increased by approximately 30% when administered with oral ranitidine at a dose of 150 mg twice daily. Monitor patients for excessive or prolonged sedation.
Carcinogenesis, Mutagenesis, Impairment of Fertility: There was no indication of tumorigenic or carcinogenic effects in life-span studies in mice and rats at dosages up to 2,000 mg/kg/day.
Ranitidine was not mutagenic in standard bacterial tests (Salmonella, Escherichia coli) for mutagenicity at concentrations up to the maximum recommended for these assays. In a dominant lethal assay, a single oral dose of 1,000 mg/kg to male rats was without effect on the outcome of 2 matings per week for the next 9 weeks.
Pregnancy:Teratogenic Effects: Pregnancy Category B. Reproduction studies have been performed in rats and rabbits at doses up to 160 times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to Ranitidine Tablets, USP. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers: Ranitidine is secreted in human milk. Caution should be exercised when Ranitidine Tablets, USP is administered to a nursing mother.
Pediatric Use: The safety and effectiveness of Ranitidine Tablets, USP have been established in the age-group of 1 month to 16 years for the treatment of duodenal and gastric ulcers, gastroesophageal reflux disease and erosive esophagitis, and the maintenance of healed duodenal and gastric ulcer. Use of Ranitidine Tablets, USP in this age-group is supported by adequate and well-controlled studies in adults, as well as additional pharmacokinetic data in pediatric patients and an analysis of the published literature (see CLINICAL PHARMACOLOGY: Pediatrics and DOSAGE AND ADMINISTRATION: Pediatric Use).
Safety and effectiveness in pediatric patients for the treatment of pathological hypersecretory conditions or the maintenance of healing of erosive esophagitis have not been established.
Safety and effectiveness in neonates (less than 1 month of age) have not been established (see CLINICAL PHARMACOLOGY: Pediatrics)
Geriatric Use: Of the total number of subjects enrolled in US and foreign controlled clinical trials of oral formulations of Ranitidine Tablets, USP, for which there were subgroup analyses, 4,197 were 65 and over, while 899 were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, caution should be exercised in dose selection, and it may be useful to monitor renal function (see CLINICAL PHARMACOLOGY: Pharmacokinetics: Geriatrics and DOSAGE AND ADMINISTRATION: Dosage Adjustment for Patients With Impaired Renal Function).
ADVERSE REACTIONS
The following have been reported as events in clinical trials or in the routine management of patients treated with Ranitidine Tablets, USP. The relationship to therapy with Ranitidine Tablets, USP has been unclear in many cases. Headache, sometimes severe, seems to be related to administration of Ranitidine Tablets, USP.
Central Nervous System: Rarely, malaise, dizziness, somnolence, insomnia, and vertigo. Rare cases of reversible mental confusion, agitation, depression, and hallucinations have been reported, predominantly in severely ill elderly patients. Rare cases of reversible blurred vision suggestive of a change in accommodation have been reported. Rare reports of reversible involuntary motor disturbances have been received.
Cardiovascular: As with other H 2-blockers, rare reports of arrhythmias such as tachycardia, bradycardia, atrioventricular block, and premature ventricular beats.
Gastrointestinal: Constipation, diarrhea, nausea/vomiting, abdominal discomfort/pain, and rare reports of pancreatitis.
Hepatic: There have been occasional reports of hepatocellular, cholestatic, or mixed hepatitis, with or without jaundice. In such circumstances, ranitidine should be immediately discontinued. These events are usually reversible, but in rare circumstances death has occurred. Rare cases of hepatic failure have also been reported. In normal volunteers, SGPT values were increased to at least twice the pretreatment levels in 6 of 12 subjects receiving 100 mg intravenously 4 times daily for 7 days, and in 4 of 24 subjects receiving 50 mg intravenously 4 times daily for 5 days.
Musculoskeletal: Rare reports of arthralgias and myalgias.
Hematologic: Blood count changes (leukopenia, granulocytopenia, and thrombocytopenia) have occurred in a few patients. These were usually reversible. Rare cases of agranulocytosis, pancytopenia, sometimes with marrow hypoplasia, and aplastic anemia and exceedingly rare cases of acquired immune hemolytic anemia have been reported.
Endocrine: Controlled studies in animals and man have shown no stimulation of any pituitary hormone by Ranitidine Tablets, USP and no antiandrogenic activity, and cimetidine-induced gynecomastia and impotence in hypersecretory patients have resolved when Ranitidine Tablets, USP has been substituted. However, occasional cases of impotence and loss of libido have been reported in male patients receiving Ranitidine Tablets, USP, but the incidence did not differ from that in the general population.
Rare cases of breast symptoms and conditions, including galactorrhea and gynecomastia, have been reported in both males and females.
Integumentary: Rash, including rare cases of erythema multiforme. Rare cases of alopecia and vasculitis.
Respiratory: A large epidemiological study suggested an increased risk of developing pneumonia in current users of histamine-2-receptor antagonists (H 2RAs) compared to patients who had stopped H 2RA treatment, with an observed adjusted relative risk of 1.63 (95% CI, 1.07 to 2.48). However, a causal relationship between use of H 2RAs and pneumonia has not been established.
Other: Rare cases of hypersensitivity reactions (e.g., bronchospasm, fever, rash, eosinophilia), anaphylaxis, angioneurotic edema, acute interstitial nephritis, and small increases in serum creatinine.
OVERDOSAGE
There has been limited experience with overdosage. Reported acute ingestions of up to 18 g orally have been associated with transient adverse effects similar to those encountered in normal clinical experience (seeADVERSE REACTIONS). In addition, abnormalities of gait and hypotension have been reported.
When overdosage occurs, the usual measures to remove unabsorbed material from the gastrointestinal tract, clinical monitoring, and supportive therapy should be employed.
Studies in dogs receiving dosages of Ranitidine Tablets, USP in excess of 225 mg/kg/day have shown muscular tremors, vomiting, and rapid respiration. Single oral doses of 1,000 mg/kg in mice and rats were not lethal. Intravenous LD 50 values in mice and rats were 77 and 83 mg/kg, respectively.
DOSAGE AND ADMINISTRATION
Active Duodenal Ulcer: The current recommended adult oral dosage of Ranitidine Tablets, USP for duodenal ulcer is 150 mg twice daily. An alternative dosage of 300 mg once daily after the evening meal or at bedtime can be used for patients in whom dosing convenience is important. The advantages of one treatment regimen compared to the other in a particular patient population have yet to be demonstrated (see Clinical Trials: Active Duodenal Ulcer). Smaller doses have been shown to be equally effective in inhibiting gastric acid secretion in US studies, and several foreign trials have shown that 100 mg twice daily is as effective as the 150-mg dose. Antacid should be given as needed for relief of pain (see CLINICAL PHARMACOLOGY: Pharmacokinetics).
Maintenance of Healing of Duodenal Ulcers: The current recommended adult oral dosage is 150 mg at bedtime.
Pathological Hypersecretory Conditions (such as Zollinger-Ellison syndrome): The current recommended adult oral dosage is 150 mg twice daily. In some patients it may be necessary to administer Ranitidine Tablets, USP 150-mg doses more frequently. Dosages should be adjusted to individual patient needs, and should continue as long as clinically indicated. Dosages up to 6 g/day have been employed in patients with severe disease.
Benign Gastric Ulcer: The current recommended adult oral dosage is 150 mg twice daily.
Maintenance of Healing of Gastric Ulcers: The current recommended adult oral dosage is 150 mg at bedtime.
GERD: The current recommended adult oral dosage is 150 mg twice daily.
Erosive Esophagitis: The current recommended adult oral dosage is 150 mg four times daily.
Maintenance of Healing of Erosive Esophagitis: The current recommended adult oral dosage is 150 mg twice daily.
Pediatric Use: The safety and effectiveness of Ranitidine Tablets, USP have been established in the age-group of 1 month to 16 years. There is insufficient information about the pharmacokinetics of Ranitidine Tablets, USP in neonatal patients (less than 1 month of age) to make dosing recommendations.
The following 3 subsections provide dosing information for each of the pediatric indications.
Treatment of Duodenal and Gastric Ulcers: The recommended oral dose for the treatment of active duodenal and gastric ulcers is 2 to 4 mg/kg twice daily to a maximum of 300 mg/day. This recommendation is derived from adult clinical studies and pharmacokinetic data in pediatric patients.
Maintenance of Healing of Duodenal and Gastric Ulcers: The recommended oral dose for the maintenance of healing of duodenal and gastric ulcers is 2 to 4 mg/kg once daily to a maximum of 150 mg/day. This recommendation is derived from adult clinical studies and pharmacokinetic data in pediatric patients.
Treatment of GERD and Erosive Esophagitis: Although limited data exist for these conditions in pediatric patients, published literature supports a dosage of 5 to 10 mg/kg/day, usually given as two divided doses.
Dosage Adjustment for Patients With Impaired Renal Function: On the basis of experience with a group of subjects with severely impaired renal function treated with Ranitidine Tablets, USP, the recommended dosage in patients with a creatinine clearance <50 mL/min is 150 mg every 24 hours. Should the patient's condition require, the frequency of dosing may be increased to every 12 hours or even further with caution. Hemodialysis reduces the level of circulating ranitidine. Ideally, the dosing schedule should be adjusted so that the timing of a scheduled dose coincides with the end of hemodialysis.
Elderly patients are more likely to have decreased renal function, therefore caution should be exercised in dose selection, and it may be useful to monitor renal function (see CLINICAL PHARMACOLOGY: Pharmacokinetics: Geriatrics and PRECAUTIONS: Geriatric Use).
HOW SUPPLIED
Ranitidine Tablets, USP 150 mg (ranitidine HCl equivalent to 150 mg of ranitidine) are supplied as orange, round, biconvex aqueous film-coated tablets debossed “IP 253” on one side and plain on the reverse.
Bottles of 60: NDC: 53746-253-60
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature] in a dry place. Dispense in a tight, light resistant container as defined in the USP. Protect from light. Replace cap securely after each opening.
-
DermacinRx Penetral Cream
Drug Facts
Uses
For the temporary relief of minor aches and pains of muscles and joints associated with arthritis, strains, and sprains.
Warnings
For external use only
This is not a face cream. Do not apply to the face.
Do not use if allergic to chili peppers or if past allergic reaction to capsaicin.
Do not apply to wounds or damaged, broken, sunburned, chapped or irritated skin.
Do not bandage tightly.
Do not apply within 1 hour before or after bath, shower, hot tub, sauna or vigorous exercise. Warm water, perspiration or open pores can intensify the impact of this product and cause a burning sensation.
Do not use with heating pad, hot water bottle or other source of heat. Doing so can increase risk of serious burns.
When using this product do not get into eyes and avoid contact with other mucous membranes. If contact occurs or if pain, discomfort or skin redness occurs, continually rinse with cool water and seek medical help.
Discontinue use and consult a doctor if condition worsens or if symptoms persist for more than 7 days or clear up and occur again within a few days. Stop using product immediately and get medical attention if experiencing burning, pain, swelling, or blistering of the skin. Rare cases of severe burning or blistering have been reported.
If pregnant, breast-feeding, or any medical conditions exist, ask a health professional before use.
Keep out of reach of children and pets. If swallowed, get medical help or contact a Poison Control Center right away. If inhaled, remove to fresh air. If breathing is difficult, get medical attention immediately.
Directions
- Rotate pump’s spout counter-clockwise slightly to unlock; clockwise to lock.
- Before using on children under 18 years of age consult a physician.
- Apply sparingly to affected area not more than 4 times daily. However, for first use, apply to small area to test for sensitivity or skin reaction.
- Gently massage into the skin until fully absorbed.
- Wash hands with soap and water thoroughly after each application to avoid spreading to the eyes or other sensitive mucous membranes.
WARNING: FLAMMABLE PRODUCT
Store in a cool well ventilated area away from heat. Keep away from sparks or open flame.
Inactive ingredients
Acrylates Copolymer, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis (Aloe Vera) Leaf Juice, Aqua (Purified Water), Arnica Montana Flower Extract, Ethyl Alcohol, Ethyl Menthane Carboxamide, Ethylhexylglycerin, Euterpe Oleracea (Acai) Fruit Oil, Glycerin, Hydroxyethyl Behenamidopropyl Dimonium Chloride, Lauryl Laurate, Linum Usitatissimum (Flax) Seed Oil, Mentha Piperita (Peppermint) Oil, Menthyl Lactate, Methyl Diisopropyl Propionamide, Phenoxyethanol, PPG-2 Hydroxyethyl Cocamide, Rosmarinus Officinals (Rosemary) Oil, Triethanolamine.
- DermasilkRx Anodynexa Pak- carton
-
INGREDIENTS AND APPEARANCE
DERAMSILKRX ANODYNEXA PAK
diclofenac sodium delayed release tablets, ranitidine tablets, capsaicin cream kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69329-310 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69329-310-00 1 in 1 PACKAGE Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, PLASTIC 60 Part 2 1 BOTTLE, PLASTIC 60 Part 3 1 TUBE 237 mL Part 1 of 3 DICLOFENAC SODIUM
diclofenac sodium tablet, delayed releaseProduct Information Item Code (Source) NDC: 0228-2551 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DICLOFENAC SODIUM (UNII: QTG126297Q) (DICLOFENAC - UNII:144O8QL0L1) DICLOFENAC SODIUM 75 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM ALGINATE (UNII: C269C4G2ZQ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STEARIC ACID (UNII: 4ELV7Z65AP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white Score no score Shape ROUND Size 9mm Flavor Imprint Code R;551 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 KIT 1 NDC: 0228-2551-06 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074514 03/26/1996 Part 2 of 3 RANITIDINE
ranitidine tabletProduct Information Item Code (Source) NDC: 53746-253 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RANITIDINE HYDROCHLORIDE (UNII: BK76465IHM) (RANITIDINE - UNII:884KT10YB7) RANITIDINE 150 mg Inactive Ingredients Ingredient Name Strength COLLOIDAL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color orange Score no score Shape ROUND (Biconvex) Size 9mm Flavor Imprint Code IP;253 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 KIT 1 NDC: 53746-253-60 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077824 12/16/2009 Part 3 of 3 PENETRAL CREAM
capsaicin creamProduct Information Item Code (Source) NDC: 69329-323 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSICUM OLEORESIN (UNII: UW86K581WY) (CAPSICUM OLEORESIN - UNII:UW86K581WY) CAPSAICIN 0.25 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 1 in 1 KIT 1 NDC: 69329-323-01 237 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 06/09/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074514 06/12/2015 Labeler - Patchwerx Labs, Inc. (079584480)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
