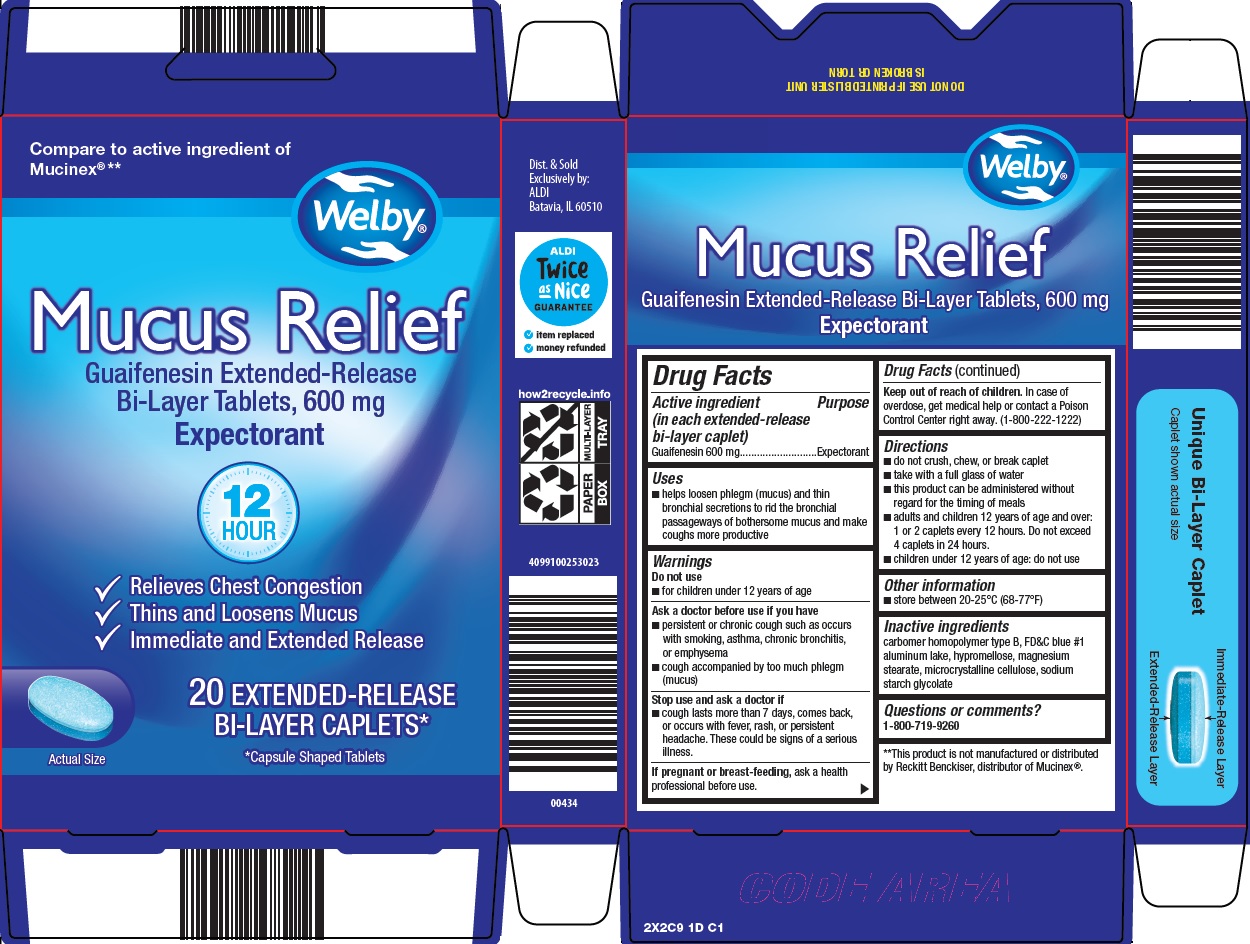
Aldi Inc. Mucus Relief Drug Facts
welby mucus relief by
Drug Labeling and Warnings
welby mucus relief by is a Otc medication manufactured, distributed, or labeled by Aldi Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
WELBY MUCUS RELIEF- guaifenesin tablet, multilayer, extended release
Aldi Inc
----------
Aldi Inc. Mucus Relief Drug Facts
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
Warnings
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
Directions
- do not crush, chew, or break tablet
- take with a full glass of water
- this product can be administered without regard for the timing of meals
- adults and children 12 years of age and over: 1 or 2 tablets every 12 hours. Do not exceed 4 tablets in 24 hours.
- children under 12 years of age: do not use
| WELBY MUCUS RELIEF
guaifenesin tablet, multilayer, extended release |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Aldi Inc (944259522) |
Revised: 2/2025
Document Id: ea4c9ccd-8387-4a7d-bab1-8fb23958ec23
Set id: 1b6622a9-2e3b-418f-a747-f79152d36a27
Version: 3
Effective Time: 20250219
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.