ANTI-DIARRHEAL- loperamide hcl tablet
Anti-diarrheal by
Drug Labeling and Warnings
Anti-diarrheal by is a Otc medication manufactured, distributed, or labeled by Chain Drug Consortium, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each caplet)
- Purpose
- Use
-
Warnings
Allergy alert: Do not use if you have ever had a rash or other allergic reaction to loperamide HCl.
Heart alert: Taking more than directed can cause serious heart problems or death.Ask a doctor or pharmacist before use if you are
taking a prescription drug. Loperamide may interact with certain prescription drugs.
When using this product,
tiredness, drowsiness, or dizziness may occur. Be careful when driving or operating machinery.
-
Directions
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- find right dose on chart. If possible, use weight to dose; otherwise, use age.
adults and children 12 years and over 2 caplets after the first loose stool; 1 caplet after each subsequent
loose stool; but no more than 4 caplets in 24 hourschildren 9-11 years (60-95 lbs) 1 caplet after the first loose stool; 1/2 caplet after each subsequent
loose stool; but no more than 3 caplets in 24 hourschildren 6-8 years (48-59 lbs) 1 caplet after the first loose stool; 1/2 caplet after each subsequent
loose stool; but no more than 2 caplets in 24 hourschildren 2-5 years (34-47 lbs) ask a doctor children under 2 years (up to 33 lbs) do not use
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
*COMPARE TO THE ACTIVE INGREDIENT IN IMODIUM® A-D
Premier
Value®Anti-diarrheal
Loperamide HCl Tablets, 2 mg
ANTI-DIARRHEALControls the symptoms
of diarrhea6 Caplets
actual size
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
*This product is not manufactured or distributed by Johnson & Johnson Corporation, owner of the registered trademark Imodium® A-D.
50844 ORG061937545
Distributed by:
Pharmacy Value Alliance, LLC
407 East Lancaster Avenue, Wayne, PA 19087
INDEPENDENTLY TESTED
PV
SATISFACTION GUARANTEED
If for any reason you are not satisfied with this product, please return it to the store where purchased for a full refund.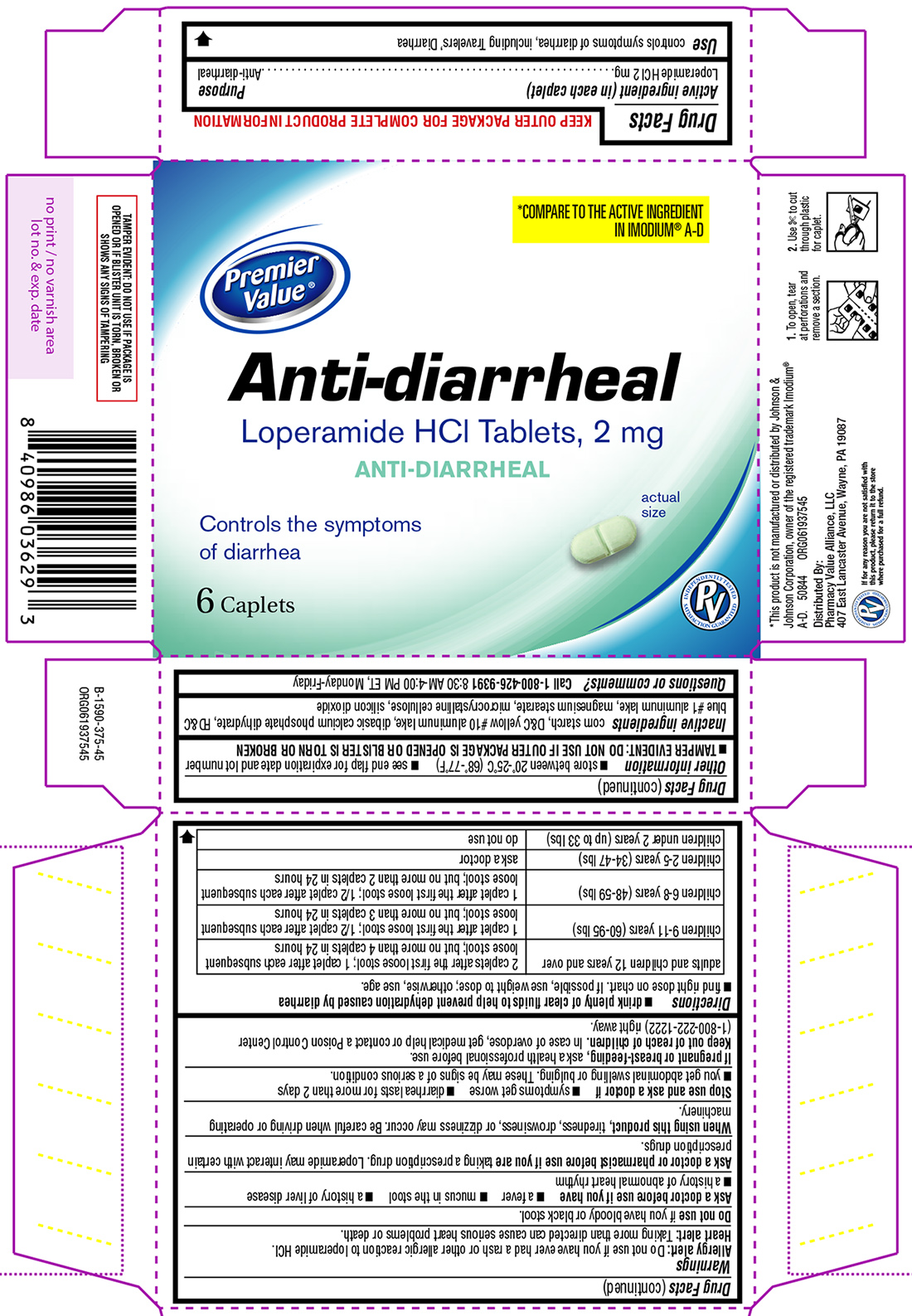
Premier Value 44-375
-
INGREDIENTS AND APPEARANCE
ANTI-DIARRHEAL
loperamide hcl tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 68016-301 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LOPERAMIDE HYDROCHLORIDE (UNII: 77TI35393C) (LOPERAMIDE - UNII:6X9OC3H4II) LOPERAMIDE HYDROCHLORIDE 2 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C BLUE NO. 1--ALUMINUM LAKE (UNII: J9EQA3S2JM) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color GREEN Score 2 pieces Shape OVAL Size 10mm Flavor Imprint Code 44;375 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68016-301-06 1 in 1 CARTON 12/06/2019 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 68016-301-18 3 in 1 CARTON 12/06/2019 2 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC: 68016-301-24 4 in 1 CARTON 12/06/2019 3 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076497 12/06/2019 Labeler - Chain Drug Consortium (101668460) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 MANUFACTURE(68016-301) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 PACK(68016-301) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 PACK(68016-301) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 PACK(68016-301) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 PACK(68016-301)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.