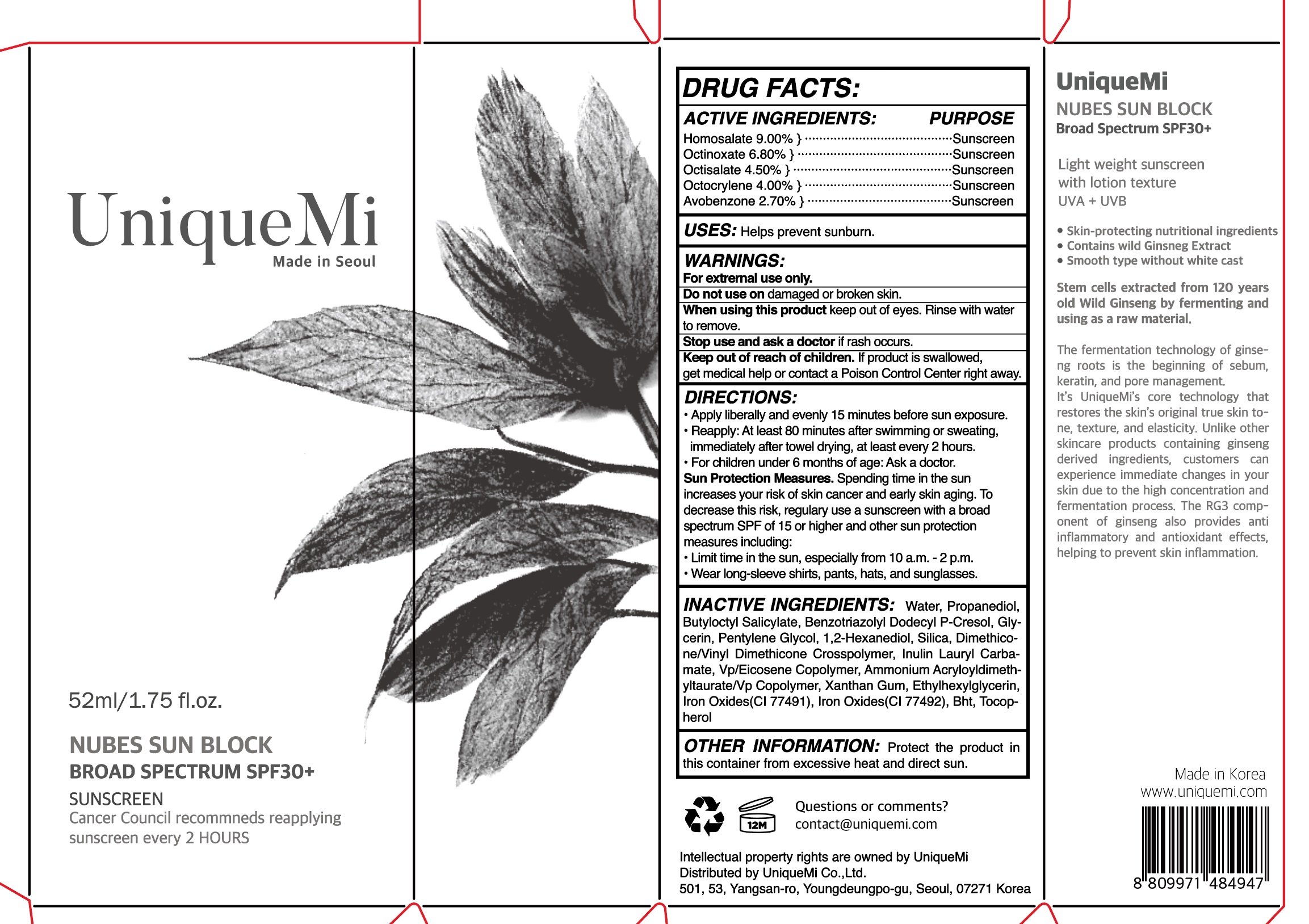
NUBES SUN BLOCK- homosalate, octinoxate, octocrylene,, avobenzone cream
Nubes SUN BLOCK by
Drug Labeling and Warnings
Nubes SUN BLOCK by is a Otc medication manufactured, distributed, or labeled by UNIQUEMI, Kolmar Korea Co., Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
ACTIVE INGREDIENT
Homosalate 9.00% } ·········································Sunscreen
Octinoxate 6.80% } ···········································Sunscreen
Octisalate 4.50% } ············································Sunscreen
Octocrylene 4.00% } ·········································Sunscreen
Avobenzone 2.70% } ········································Sunscreen
-
INACTIVE INGREDIENT
Water, Propanediol, Butyloctyl Salicylate, Benzotriazolyl Dodecyl P-Cresol, Glycerin, Pentylene Glycol, 1,2-Hexanediol, Silica, Dimethicone/Vinyl Dimethicone Crosspolymer, Inulin Lauryl Carbamate, Vp/Eicosene Copolymer, Ammonium Acryloyldimethyltaurate/Vp Copolymer, Xanthan Gum, Ethylhexylglycerin,Iron Oxides(CI 77491), Iron Oxides(CI 77492), Bht, Tocophero
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS & USAGE
Apply liberally and evenly 15 minutes before sun exposure.
Reapply: At least 80 minutes after swimming or sweating,
immediately after towel drying, at least every 2 hours.
For children under 6 months of age: Ask a doctor.
Sun Protection Measures. Spending time in the sun
increases your risk of skin cancer and early skin aging. To
decrease this risk, regulary use a sunscreen with a broad
spectrum SPF of 15 or higher and other sun protection
measures including:
Limit time in the sun, especially from 10 a.m. - 2 p.m.
Wear long-sleeve shirts, pants, hats, and sunglasses.
- WARNINGS
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NUBES SUN BLOCK
homosalate, octinoxate, octocrylene,, avobenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 84514-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 9 g in 100 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 6.8 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.7 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 4 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 84514-0001-1 52 mL in 1 CONTAINER; Type 0: Not a Combination Product 06/25/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/25/2024 Labeler - UNIQUEMI (690393808) Registrant - UNIQUEMI (690393808) Establishment Name Address ID/FEI Business Operations UNIQUEMI 690393808 label(84514-0001) Establishment Name Address ID/FEI Business Operations Kolmar Korea Co., Ltd 963271745 manufacture(84514-0001)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.