TRAMADOL HYDROCHLORIDE capsule, extended release
Tramadol Hydrochloride by
Drug Labeling and Warnings
Tramadol Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Medsource Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Tramadol Hydrochloride Extended-Release safely and effectively. See full prescribing information for Tramadol Hydrochloride Extended-Release.
Tramadol Hydrochloride Extended-Release (tramadol hydrochloride) Extended-Release Capsules
Initial U.S. Approval: 1995INDICATIONS AND USAGE
Tramadol Hydrochloride Extended-Release is an opioid agonist indicated for the management of moderate to moderately severe chronic pain in adults who require around-the-clock treatment of their pain for an extended period of time. ( 1)
DOSAGE AND ADMINISTRATION
- Tramadol Hydrochloride Extended-Release must be swallowed whole, and must not be split, chewed, dissolved or crushed. ( 2.1)
- Do not exceed a daily dose of 300 mg tramadol. Do not use with other tramadol products. ( 2.1)
- Adults not on tramadol Immediate-Release (IR): Initiate Tramadol Hydrochloride Extended-Release at a dose of 100 mg once daily, then titrate up by 150 mg, 200 mg and 300 mg, every 5 days according to need and tolerance. ( 2.1)
- Adults on tramadol IR: Calculate total 24-hr IR dose, initiate Tramadol Hydrochloride Extended-Release at a dose rounded down to next lower dose; then adjust dose according to need and tolerance. ( 2.1)
- Patients >65 years of age: Initiate dosing cautiously; use even greater caution in patients >75 years. ( 2.3)
- May be taken without regard to meals. ( 12.3)
DOSAGE FORMS AND STRENGTHS
Extended-Release Capsules: 150 mg ( 3)
CONTRAINDICATIONS
- Patients who have previously demonstrated hypersensitivity to tramadol, any other component of this product or opioids. ( 4)
- Patients with significant respiratory depression in unmonitored settings or the absence of resuscitative equipment. ( 4)
- Patients with acute or severe bronchial asthma or hypercapnia in unmonitored settings or the absence of resuscitative equipment. ( 4)
- All other opioid contraindications, including intoxication with alcohol, hypnotics, narcotics, centrally acting analgesics, opioids or psychotropic drugs. ( 4)
WARNINGS AND PRECAUTIONS
- Seizures: Can occur within the recommended dose range. Concomitant use with certain other drugs may increase the seizure risk. Risk of convulsions may increase in patients with epilepsy, history of seizures, and with a recognized risk for seizure. ( 5.1, 5.9)
- Suicide: Do not prescribe for suicidal or addiction-prone patients. ( 5.2)
- Serotonin Syndrome: May be life-threatening. Can occur with tramadol alone or with concomitant use of other serotonergic drugs or those that inhibit metabolism of tramadol. ( 5.3. 5.9)
- Anaphylactoid Reactions: Serious and rarely fatal anaphylactoid reactions have occurred. Increased risk in patients with history of anaphylactoid reactions to other opioids. ( 5.4)
- Hypersensitivity reactions: Serious and rarely fatal anaphylactoid reactions have occurred, often after the first dose. Other reactions include pruritus, hives, bronchospasm, angioedema, TEN, SJS. ( 5.4)
- Respiratory Depression: Administer cautiously in patients at risk for respiratory depression. ( 5.5)
- CNS Depression: Use with caution and in reduced dosages in patients taking CNS depressants and in patients at risk for CNS depression. Patients should not consume alcohol-containing beverages while using Tramadol Hydrochloride Extended-Release ( 5.6, 5.12)
- Increased Intracranial Pressure or Head Trauma: Use with caution in patients with increased intracranial pressure or head injury. ( 5.7)
- Ambulatory Patients: Tramadol may impair mental and/or physical abilities required for potentially hazardous tasks. ( 5.8)
- Tapering may reduce withdrawal symptoms. ( 5.10)
- Misuse, Abuse, and Diversion: Tramadol can be abused in a manner similar to other opioid agonists, legal or illicit. ( 5.11)
- Overdosage can cause CNS and respiratory depression and death. ( 5.13)
- Tramadol Hydrochloride Extended-Release may complicate clinical assessment of acute abdominal conditions. (5.14)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 10% and twice placebo) are nausea, constipation, dry mouth, somnolence, dizziness, and vomiting. ( 6)
To report SUSPECTED ADVERSE REACTIONS, contact Vertical at (877) 958-3784 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- SSRI/SNRI antidepressants or anorectics, TCA antidepressants, other tricyclic compounds, other opioids, MAOIs, neuroleptics or other drugs that lower seizure threshold: Risk of seizures increased with concomitant use of tramadol. ( 7.1)
- CYP2D6 and/or CYP3A4 Inhibitors: May result in increased tramadol concentrations. ( 7.2)
- Serotonergic Drugs, Triptans, and CNS Depressants: Enhanced risk of adverse reactions. ( 7.3, 7.4, and 7.5)
- Carbamazepine: Reduces analgesic effects of tramadol. ( 7.6)
- Quinidine: May result in increased concentration of tramadol and reduced concentrations of its active metabolite, M1. ( 7.7)
- Digoxin and Warfarin: Rare reports of digoxin toxicity; altered warfarin effect and elevation of prothrombin time. ( 7.8)
USE IN SPECIFIC POPULATIONS
- Pregnancy: Use only if benefit outweighs risk to the fetus. Neonatal seizures and withdrawal syndrome, fetal death and still birth have occurred. ( 8.1)
- Labor and Delivery: Tramadol crosses the placenta, and should not be used prior to or during labor unless the potential benefits outweigh the risks. Long term use during pregnancy can cause dependence and withdrawal symptoms in the newborn. ( 8.2)
- Nursing mothers: Effect on child not studied. Tramadol Hydrochloride Extended-Release should not be used. ( 8.3)
- Pediatrics: Safety and effectiveness of tramadol has not been established in patients under 18 years. Use in this population is not recommended. ( 8.4)
- Elderly: Use with caution, in particular in patients older than 75 years. ( 8.5)
- Severe renal impairment: Tramadol Hydrochloride Extended-Release should not be used. ( 8.6)
- Severe hepatic impairment: Tramadol Hydrochloride Extended-Release should not be used. ( 8.7)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2014
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Considerations
2.2 Patients Not Currently on Tramadol Immediate-Release Products
2.3 Patients Currently on Tramadol Immediate-Release Products
2.4 Patients 65 Years of Age and Older
2.5 Patients with Renal Impairment
2.6 Patients with Hepatic Impairment
2.7 Discontinuation of Treatment
2.8 Food Effects
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Seizure Risk
5.2 Suicide Risk
5.3 Serotonin Syndrome Risk
5.4 Anaphylactoid Reactions
5.5 Respiratory Depression
5.6 Interaction With Central Nervous System (CNS) Depressants, Including Alcohol and Drugs of Abuse
5.7 Patients with Increased Intracranial Pressure or Head Trauma
5.8 Use in Ambulatory Patients
5.9 Use With MAO Inhibitors and SSRIs
5.10 Withdrawal Symptoms
5.11 Misuse, Abuse and Diversion of Opioids
5.12 Risk of Overdosage
5.13 Acute Abdominal Conditions
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
7 DRUG INTERACTIONS
7.1 Drugs Affecting Seizure Threshold
7.2 CYP2D6 and/or CYP3A4 inhibitors
7.3 Serotonergic Drugs
7.4 Triptans
7.5 Interaction With Central Nervous System (CNS) Depressants
7.6 Quinidine
7.7 Digoxin and Warfarins
7.8 CYP3A4 Inducers
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients with Renal Impairment
8.7 Patients with Hepatic Impairment
9 DRUG ABUSE and DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
10.1 Human Experience
10.2 Management of Overdose
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NON-CLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Considerations
Tramadol Hydrochloride Extended-Release is an extended-release formulation intended for once a day dosing in adults aged 18 years and older. The tablets must be swallowed whole with liquid and must not be split, chewed, dissolved or crushed. Chewing, crushing or splitting the tablet could result in the uncontrolled delivery of tramadol, in overdose and death [see WARNINGS AND PRECAUTIONS ( 5.11), DRUG ABUSE AND DEPENDENCE ( 9), and OVERDOSE ( 10.1)].
Do not administer Tramadol Hydrochloride Extended-Release at a dose exceeding 300 mg per day. Do not use Tramadol Hydrochloride Extended-Release more than once daily or concomitantly with other tramadol products [see WARNINGS AND PRECAUTIONS ( 5.12)].
2.2 Patients Not Currently on Tramadol Immediate-Release Products
Initiate treatment with Tramadol Hydrochloride Extended-Release at a dose of 100 mg once daily and titrated up as necessary to 150 mg, 200 mg and 300 mg every five days to achieve a balance between relief of pain and tolerability.
2.3 Patients Currently on Tramadol Immediate-Release Products
Calculate the 24-hour tramadol IR dose and initiate a total daily dose of Tramadol Hydrochloride Extended-Release rounded down to the next lowest 100 mg increment. The dose may subsequently be individualized according to patient need. Due to limitations in flexibility of dose selection with Tramadol Hydrochloride Extended-Release, some patients maintained on tramadol IR products may not be able to convert to Tramadol Hydrochloride Extended-Release.
2.4 Patients 65 Years of Age and Older
Initiate dosing of an elderly patient (over 65 years of age) should be initiated cautiously, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function and of concomitant disease or other drug therapy. Tramadol Hydrochloride Extended-Release should be administered with even greater caution in patients over 75 years, due to the greater frequency of adverse events seen in this population.
2.5 Patients with Renal Impairment
The limited availability of dose strengths and once daily dosing of Tramadol Hydrochloride Extended-Release do not permit the dosing flexibility required for safe use in patients with severe renal impairment. Do not use Tramadol Hydrochloride Extended-Release in patients with creatinine clearance less than 30 mL/min [see USE IN SPECIFIC POPULATIONS ( 8.6) and CLINICAL PHARMACOLOGY ( 12.3) ].
2.6 Patients with Hepatic Impairment
The limited availability of dose strengths and once daily dosing of tramadol hydrochloride extended-release capsules do not permit the dosing flexibility required for safe use in patients with severe hepatic impairment. Do not use Tramadol Hydrochloride Extended-Release in patients with severe hepatic impairment (Child-Pugh Class C) [see USE IN SPECIFIC POPULATIONS ( 8.7) and CLINICAL PHARMACOLOGY ( 12.3) ].
2.7 Discontinuation of Treatment
Withdrawal symptoms may occur if Tramadol Hydrochloride Extended-Release is discontinued abruptly. Clinical experience with tramadol suggests that withdrawal symptoms may be reduced by tapering Tramadol Hydrochloride Extended-Release [see WARNINGS AND PRECAUTIONS ( 5.10) and DRUG ABUSE AND DEPENDENCE ( 9.3)] .
2.8 Food Effects
Tramadol Hydrochloride Extended-Release may be taken without regard to food [see CLINICAL PHARMACOLOGY ( 12.3)].
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Tramadol Hydrochloride Extended-Release is contraindicated in patients who have previously demonstrated hypersensitivity to tramadol, any other component of Tramadol Hydrochloride Extended-Release, or opioids. Reactions range from pruritis to fatal anaphylactoid reactions [see WARNINGS AND PRECAUTIONS ( 5.4)].
Tramadol Hydrochloride Extended-Release is contraindicated in patients with significant respiratory depression in unmonitored settings or the absence of resuscitative equipment.
Tramadol Hydrochloride Extended-Release is contraindicated in patients with acute or severe bronchial asthma or hypercapnia in unmonitored settings or the absence of resuscitative equipment.
-
5 WARNINGS AND PRECAUTIONS
5.1 Seizure Risk
Seizures have been reported in patients receiving tramadol within the recommended dosage range. Spontaneous post-marketing reports indicate that seizure risk is increased with doses of tramadol above the recommended range. Concomitant use of tramadol increases the seizure risk in patients taking: [see DRUG INTERACTIONS ( 7.1, 7.2)]
- Selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors (SNRIs) antidepressants or anorectics,
- Tricyclic antidepressants (TCAs), and other tricyclic compounds (e.g., cyclobenzaprine, promethazine, etc.),
- Other opioids,
- MAO inhibitors [see WARNINGS AND PRECAUTIONS ( 5.9) and DRUG INTERACTIONS ( 7.1)],
- Neuroleptics, or
- Other drugs that reduce the seizure threshold.
Risk of seizures may also increase in patients with epilepsy, those with a history of seizures, or in patients with a recognized risk for seizure (such as head trauma, metabolic disorders, alcohol and drug withdrawal, CNS infections).
In tramadol overdose, naloxone administration may increase the risk of seizure.
5.2 Suicide Risk
- Do not prescribe Tramadol Hydrochloride Extended-Release for patients who are suicidal or addiction-prone. Consideration should be given to the use of non-narcotic analgesics in patients who are suicidal or depressed [see DRUG ABUSE AND DEPENDENCE ( 9.2) ].
- Prescribe Tramadol Hydrochloride Extended-Release with caution for patients with a history of misuse and/or are taking CNS-active drugs including tranquilizers or antidepressant drugs, or alcohol in excess, and patients who suffer from emotional disturbance or depression [see DRUG INTERACTIONS ( 7.4)].
- Tell your patients not to exceed the recommended dose and to limit their intake of alcohol [see DOSAGE AND ADMINISTRATION ( 2.1) and WARNINGS AND PRECAUTIONS ( 5.6)] .
5.3 Serotonin Syndrome Risk
The development of a potentially life-threatening serotonin syndrome may occur with use of tramadol products, including Tramadol Hydrochloride Extended-Release , particularly with concomitant use of serotonergic drugs such as SSRIs, SNRIs, TCAs, MAOIs and triptans, with drugs which impair metabolism of serotonin (including MAOIs) and with drugs which impair metabolism of tramadol (CYP2D6 and CYP3A4 inhibitors). This may occur within the recommended dose. [see CLINICAL PHARMACOLOGY ( 12.3)] .
Serotonin syndrome may include mental-status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination) and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
5.4 Anaphylactoid Reactions
Serious and rarely fatal anaphylactoid reactions have been reported in patients receiving therapy with tramadol. When these events do occur it is often following the first dose. Other reported allergic reactions include pruritus, hives, bronchospasm, angioedema, toxic epidermal necrolysis and Stevens-Johnson syndrome. Patients with a history of anaphylactoid reactions to codeine and other opioids may be at increased risk and therefore should not receive Tramadol Hydrochloride Extended-Release [see CONTRAINDICATIONS ( 4)].
5.5 Respiratory Depression
Administer Tramadol Hydrochloride Extended-Release cautiously in patients at risk for respiratory depression. In these patients alternative non-opioid analgesics should be considered. If large doses of tramadol are administered with anesthetic medications or alcohol, respiratory depression may result. Respiratory depression should be treated as an overdose. If naloxone is to be administered, use cautiously because it may precipitate seizures [see WARNINGS AND PRECAUTIONS ( 5.1) and OVERDOSAGE ( 10)] .
5.6 Interaction With Central Nervous System (CNS) Depressants, Including Alcohol and Drugs of Abuse
Tramadol may be expected to have additive effects when used in conjunction with alcohol, other opioids, or illicit drugs that cause central nervous system depression. Use Tramadol Hydrochloride Extended-Release with caution and in reduced dosages when administered to patients receiving CNS depressants such as alcohol, opioids, anesthetic agents, narcotics, phenothiazines, tranquilizers or sedative hypnotics. Tramadol Hydrochloride Extended-Release increases the risk of CNS and respiratory depression in these patients. Alcohol-containing beverages should not be consumed by patients using Tramadol Hydrochloride Extended-Release [see WARNINGS AND PRECAUTIONS ( 5.6), DRUG INTERACTIONS ( 7.4), and OVERDOSAGE ( 10)] .
5.7 Patients with Increased Intracranial Pressure or Head Trauma
Use Tramadol Hydrochloride Extended-Release with caution in patients with increased intracranial pressure or head injury. The respiratory depressant effects of opioids include carbon dioxide retention and secondary elevation of cerebrospinal fluid pressure, and may be markedly exaggerated in these patients. Additionally, pupillary changes (miosis) from tramadol may obscure the existence, extent, or course of intracranial pathology. Clinicians should also maintain a high index of suspicion for adverse drug reaction when evaluating altered mental status in these patients if they are receiving Tramadol Hydrochloride Extended-Release [see WARNINGS AND PRECAUTIONS ( 5.5)] .
5.8 Use in Ambulatory Patients
Tramadol Hydrochloride Extended-Release may impair the mental and or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery. Caution patients initiating therapy with Tramadol Hydrochloride Extended-Release or those whose dose has been increased to refrain from potentially hazardous activities until it is established that their mental and physical abilities are not significantly impaired.
5.9 Use With MAO Inhibitors and SSRIs
Use Tramadol Hydrochloride Extended-Release with great caution in patients taking monoamine oxidase inhibitors. Animal studies have shown increased deaths with combined administration. Concomitant use of Tramadol Hydrochloride Extended-Release with MAO inhibitors or SSRI's increases the risk of adverse reactions, including seizure and serotonin syndrome [see WARNINGS AND PRECAUTIONS ( 5.1, 5.3)] .
5.10 Withdrawal Symptoms
Withdrawal symptoms may occur if Tramadol Hydrochloride Extended-Release is discontinued abruptly. These symptoms may include: anxiety, sweating, insomnia, rigors, pain, nausea, tremors, diarrhea, upper respiratory symptoms, piloerection, and rarely hallucinations. Clinical experience with other formulations of tramadol suggests that withdrawal symptoms may be reduced by tapering Tramadol Hydrochloride Extended-Release when discontinuing tramadol therapy.
5.11 Misuse, Abuse and Diversion of Opioids
Tramadol Hydrochloride Extended-Release contains tramadol, an opioid agonist of the morphine-type. Such drugs are sought by drug abusers and people with addiction disorders and are subject to criminal diversion.
Tramadol can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when prescribing or dispensing Tramadol Hydrochloride Extended-Release in situations where the physician or pharmacist is concerned about an increased risk of misuse, abuse, or diversion.
Tramadol Hydrochloride Extended-Release could be abused by crushing, chewing, snorting, or injecting the dissolved product. These practices will result in the uncontrolled delivery of the opioid and pose a significant risk to the abuser that could result in overdose and death [see WARNINGS AND PRECAUTIONS ( 5.13), DRUG ABUSE AND DEPENDENCE ( 9), and OVERDOSAGE ( 10)] .
Concerns about abuse, addiction, and diversion should not prevent the proper management of pain. The development of addiction to opioid analgesics in properly managed patients with pain has been reported to be rare. However, data are not available to establish the true incidence of addiction in chronic pain patients.
Healthcare professionals should contact their State Professional Licensing Board, or State Controlled Substances Authority for information on how to prevent and detect abuse or diversion of this product.
5.12 Risk of Overdosage
Serious potential consequences of overdosage with Tramadol Hydrochloride Extended-Release are central nervous system depression, respiratory depression and death. In treating an overdose, primary attention should be given to maintaining adequate ventilation along with general supportive treatment [see OVERDOSAGE ( 10)].
-
6 ADVERSE REACTIONS
The following serious or otherwise important adverse reactions are described in greater detail, in other sections:
- - Seizure risk [ see WARNINGS AND PRECAUTIONS ( 5.1) ]
- - Suicide risk [ see WARNINGS AND PRECAUTIONS ( 5.2) ]
- - Serotonin syndrome [ see WARNINGS AND PRECAUTIONS ( 5.3) ]
- - Anaphylactoid and allergic reactions [ see WARNINGS AND PRECAUTIONS ( 5.4) ]
- - Respiratory depression [ see WARNINGS AND PRECAUTIONS ( 5.5) ]
- - Withdrawal symptoms [ see WARNINGS AND PRECAUTIONS ( 5.10) ]
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Tramadol Hydrochloride Extended-Release capsules were administered to a total of 1987 patients in clinical trials. These included four double-blind and one long-term, open-label study in patients with osteoarthritis of the hip and knee. A total of 812 patients were 65 years or older. Adverse reactions with doses from 100 mg to 300 mg in the four pooled, randomized, double-blind, placebo-controlled studies in patients with chronic non-malignant pain are presented in the following table (see Table 1).
Table 1: Incidence (%) of patients with adverse reaction rates ≥ 5% from four double-blind, placebo controlled studies in patients with moderate to moderately severe chronic pain by dose (N=1917). Tramadol Hydrochloride Extended-Release PLACEBO Preferred Term 100 mg
(N=429)
n (%)200 mg
(N=434)
n (%)300 mg
(N=1054)
n (%)(N=646)
n (%)Headache 99 (23.1) 96 (22.1) 200 (19.0) 128 (19.8) Nausea 69 (16.1) 93 (21.4) 265 (25.1) 37 (5.7) Somnolence 50 (11.7) 60 (13.8) 170 (16.1) 26 (4.0) Dizziness 41 (9.6) 54 (12.4) 143 (13.6) 31 (4.8) Constipation 40 (9.3) 59 (13.6) 225 (21.3) 27 (4.2) Vomiting 28 (6.5) 45 (10.4) 98 (9.3) 12 (1.9) Arthralgia 23 (5.4) 20 (4.6) 53 (5.0) 33 (5.1) Dry Mouth 20 (4.7) 36 (8.3) 138 (13.1) 22 (3.4) Sweating 18 (4.2) 23 (5.3) 71 (6.7) 4 (0.6) Asthenia 15 (3.5) 26 (6.0) 91 (8.6) 17 (2.6) Pruritus 13 (3.0) 25 (5.8) 77 (7.3) 12 (1.9) Anorexia 9 (2.1) 23 (5.3) 60 (5.7) 1 (0.2) Insomnia 9 (2.1) 9 (2.1) 53 (5.0) 11 (1.7) The following adverse reactions were reported from all chronic pain studies (N=1917). The lists below include adverse reactions not otherwise noted in Table 1.
Adverse reactions with incidence rates of 1.0% to <5.0%
Cardiac disorders: hypertension
Gastrointestinal disorders: dyspepsia, flatulence
General disorders: abdominal pain, accidental injury, chills, fever, flu syndrome, neck pain, pelvic pain
Investigations: hyperglycemia, urine abnormality
Metabolism and nutrition disorders: peripheral edema, weight loss
Musculoskeletal, connective tissue and bone disorders: myalgia
Nervous system disorders: paresthesia, tremor, withdrawal syndrome
Psychiatric disorders: agitation, anxiety, apathy, confusion, depersonalization, depression, euphoria, nervousness
Respiratory, thoracic and mediastinal disorders: bronchitis, pharyngitis, rhinitis, sinusitis
Skin and subcutaneous tissue disorders: rash
Urogenital disorders: prostatic disorder, urinary tract infection
Vascular disorders: vasodilatationAdverse reactions with incidence rates of 0.5% to <1.0% at any dose and serious adverse reactions reported in at least two patients.
Cardiac disorders: EKG abnormal, hypotension, tachycardia
Gastrointestinal disorders: gastroenteritis
General disorders: neck rigidity, viral infection
Hematologic/Lymphatic disorders; anemia, ecchymoses
Metabolism and nutrition disorders: blood urea nitrogen increased, GGT increased, gout, SGPT increased
Musculoskeletal disorders: arthritis, arthrosis, joint disorder, leg cramps
Nervous system disorders: emotional lability, hyperkinesia, hypertonia, thinking abnormal, twitching, vertigo
Respiratory disorders: pneumonia
Skin and subcutaneous tissue disorders: hair disorder, skin disorder, urticaria
Special Senses: eye disorder, lacrimation disorder
Urogenital disorders: cystitis, dysuria, sexual function abnormality, urinary retention -
7 DRUG INTERACTIONS
7.1 Drugs Affecting Seizure Threshold
Concomitant use of tramadol increases the seizure risk in patients taking SSRI/SNRI antidepressants or anorectics, TCA antidepressants and other tricyclic compounds, other opioids, MAOIs, neuroleptics or other drugs that lower the seizure threshold [see WARNINGS AND PRECAUTIONS ( 5.1) ].
7.2 CYP2D6 and/or CYP3A4 inhibitors
Tramadol is metabolized by CYP2D6 to form the active metabolite, O-desmethyl tramadol (M1). In vitro drug interaction studies in human liver microsomes indicate that concomitant administration with inhibitors of CYP2D6 such as fluoxetine, paroxetine, and amitriptyline could result in some inhibition of the metabolism of tramadol.
Tramadol is also metabolized by CYP3A4 [see CLINICAL PHARMACOLOGY ( 12.3)] . Administration of CYP3A4 inhibitors, such as ketoconazole and erythromycin with Tramadol Hydrochloride Extended-Release may affect the metabolism of tramadol leading to altered tramadol exposure.
Concomitant administration of CYP2D6 and/or CYP3A4 inhibitors, such as quinidine, fluoxetine, paroxetine and amitriptyline (CYP2D6 inhibitors), and ketoconazole and erythromycin (CYP3A4 inhibitors), may reduce metabolic clearance of tramadol increasing the risk for serious adverse events including seizures and serotonin syndrome [see CLINICAL PHARMACOLOGY ( 12.3)] .
7.3 Serotonergic Drugs
There have been post-marketing reports of serotonin syndrome with use of tramadol and SSRIs/SNRIs or MAOIs and α2-adrenergic blockers. Caution is advised when Tramadol Hydrochloride Extended-Release is co-administered with other drugs that may affect the serotonergic neurotransmitter systems, such as SSRIs, MAOIs, triptans, linezolid (an antibiotic which is a reversible non-selective MAOI), lithium, or St. John's Wort. If concomitant treatment of Tramadol Hydrochloride Extended-Release with a drug affecting the serotonergic neurotransmitter system is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases [see WARNINGS AND PRECAUTIONS ( 5.3)].
7.4 Triptans
Based on the mechanism of action of tramadol and the potential for serotonin syndrome, caution is advised when Tramadol Hydrochloride Extended-Release is co-administered with a triptan. If concomitant treatment of Tramadol Hydrochloride Extended-Release with a triptan is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases [see WARNINGS AND PRECAUTIONS ( 5.3)].
7.5 Interaction With Central Nervous System (CNS) Depressants
Tramadol Hydrochloride Extended-Release should be used with caution and in reduced dosages when administered to patients receiving CNS depressants such as opioids, anesthetic agents, narcotics, phenothiazines, tranquilizers or sedative hypnotics. Tramadol Hydrochloride Extended-Release increases the risk of CNS and respiratory depression in these patients [see WARNINGS AND PRECAUTIONS ( 5.6)].
7.6 Quinidine
Quinidine is a strong inhibitor of CYP2D6. Coadministration of quinidine with an extended-release tramadol product resulted in a 50-60% increase in tramadol exposure and a 50-60% decrease in M1 exposure. The clinical consequences of these findings are unknown. [see CLINICAL PHARMACOLOGY ( 12.3)]. In vitro drug interaction studies in human liver microsomes indicate that tramadol has no effect on quinidine metabolism.
7.7 Digoxin and Warfarins
Post-marketing surveillance of tramadol has revealed rare reports of digoxin toxicity and alteration of warfarin effect, including elevation of prothrombin times.
7.8 CYP3A4 Inducers
Administration of CYP3A4 inducers, such as carbamazepine, rifampin and St. John's Wort, with Tramadol Hydrochloride Extended-Release may affect the metabolism of tramadol leading to reduced tramadol exposure [see CLINICAL PHARMACOLOGY ( 12.3) ].
Patients taking carbamazepine, a CYP3A4 inducer, may have a significantly reduced analgesic effect of tramadol. Because carbamazepine increases tramadol metabolism and because of the seizure risk associated with tramadol, concomitant administration of Tramadol Hydrochloride Extended-Release and carbamazepine is not recommended.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects: Pregnancy Category C
There are no adequate and well-controlled studies in pregnant women. Use Tramadol Hydrochloride Extended-Release during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Neonatal seizures, neonatal withdrawal syndrome, fetal death and still birth have been reported during post-marketing reports with tramadol HCl immediate-release products. Tramadol Hydrochloride Extended-Release should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Tramadol was not teratogenic at oral dose levels up to 50 mg/kg/day (1.6-fold the maximum daily human dose (MDHD)) in rats and 100 mg/kg (approximately 6.5-fold MDHD) in rabbits during organogenesis. However, embryo-fetal lethality, reductions in fetal weight and skeletal ossification, and increased supernumerary ribs were observed at a maternal toxic dose of 140 mg/kg in mice (approximately 2.3-fold MDHD), 80 mg/kg in rats (2.6-fold MDHD) or 300 mg/kg in rabbits (approximately 19-fold MDHD).
8.2 Labor and Delivery
Tramadol Hydrochloride Extended-Release should not be used in pregnant women prior to or during labor unless the potential benefits outweigh the risks. Safe use in pregnancy has not been established. Chronic use during pregnancy may lead to physical dependence and post-partum withdrawal symptoms in the newborn [see DRUG ABUSE AND DEPENDENCE ( 9.1)] . Tramadol has been shown to cross the placenta. The mean ratio of serum tramadol in the umbilical veins compared to maternal veins was 0.83 for 40 women given tramadol during labor.
The effect of Tramadol Hydrochloride Extended-Release, if any, on the later growth, development, and functional maturation of the child is unknown.
8.3 Nursing Mothers
Tramadol Hydrochloride Extended-Release is not recommended for obstetrical preoperative medication or for post-delivery analgesia in nursing mothers because its safety in infants and newborns has not been studied. Following a single IV 100 mg dose of tramadol, the cumulative excretion in breast milk within 16 hours post-dose was 100 μg of tramadol (0.1% of the maternal dose) and 27 μg of M1. It is not known whether this drug is excreted in human milk following an oral dose.
8.4 Pediatric Use
The safety and efficacy of Tramadol Hydrochloride Extended-Release in patients under 18 years of age have not been established. The use of Tramadol Hydrochloride Extended-Release in the pediatric population is not recommended.
8.5 Geriatric Use
In general, caution should be used when selecting the dose for an elderly patient. Usually, dose administration should start at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function and of concomitant disease or other drug therapy.
Eight hundred and twelve elderly (65 years of age or older) subjects were exposed to Tramadol Hydrochloride Extended-Release in clinical trials. Of those subjects, two hundred and forty were 75 years of age and older. In general, higher incidence rates of adverse events were observed for patients older than 65 years of age compared with patients 65 years and younger, particularly for the following adverse events: nausea, constipation, somnolence, dizziness, dry mouth, vomiting, asthenia, pruritus, anorexia sweating, fatigue, weakness, postural hypotension and dyspepsia. For this reason, Tramadol Hydrochloride Extended-Release should be used with great caution in patients older than 75 years of age [see DOSAGE AND ADMINISTRATION ( 2.3)].
8.6 Patients with Renal Impairment
Tramadol Hydrochloride Extended-Release has not been studied in patients with renal impairment. Impaired renal function results in a decreased rate and extent of excretion of tramadol and its active metabolite, M1. The limited availability of dose strengths of Tramadol Hydrochloride Extended-Release does not permit the dosing flexibility required for safe use in patients with severe renal impairment. Therefore, Tramadol Hydrochloride Extended-Release should not be used in patients with severe renal impairment [see DOSAGE AND ADMINISTRATION ( 2.3), WARNINGS AND PRECAUTIONS (5.14), and CLINICAL PHARMACOLOGY ( 12.3)] .
8.7 Patients with Hepatic Impairment
Tramadol Hydrochloride Extended-Release has not been studied in patients with hepatic impairment. The limited availability of dose strengths of Tramadol Hydrochloride Extended-Release does not permit the dosing flexibility required for safe use in patients with severe hepatic impairment. Therefore, Tramadol Hydrochloride Extended-Release should not be used in patients with severe hepatic impairment [see DOSAGE AND ADMINISTRATION ( 2.3), WARNINGS AND PRECAUTIONS, (5.14), and CLINICAL PHARMACOLOGY ( 12.3)].
-
9 DRUG ABUSE and DEPENDENCE
9.1 Controlled Substance
Tramadol Hydrochloride Extended-Release is classified as a Schedule IV controlled substance by federal regulation.
9.2 Abuse
Tramadol Hydrochloride Extended-Release contains tramadol, a mu-agonist opioid. Tramadol, like other opioids used in analgesia, can be abused and is subject to criminal diversion.
Addiction is a primary, chronic, neurobiologic disease, with genetic, psychosocial, and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving. Drug addiction is a treatable disease, utilizing a multidisciplinary approach, but relapse is common.
“Drug-seeking” behavior is very common in addicts and drug abusers. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing or referral, repeated “loss” of prescriptions, tampering with prescriptions and reluctance to provide prior medical records or contact information for other treating physician(s). “Doctor shopping” to obtain additional prescriptions is common among drug abusers and people suffering from untreated addiction.
Abuse and addiction are separate and distinct from physical dependence and tolerance. Physicians should be aware that addiction may not be accompanied by concurrent tolerance and symptoms of physical dependence in all addicts. In addition, abuse of opioids can occur in the absence of true addiction and is characterized by misuse for non-medical purposes, often in combination with other psychoactive substances. Tramadol Hydrochloride Extended-Release, like other opioids, may be diverted for non-medical use. Careful record keeping of prescribing information, including quantity, frequency, and renewal requests is strongly advised.
Proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
Tramadol Hydrochloride Extended-Release is intended for oral use only. The crushed capsule poses a hazard of overdose and death. This risk is increased with concurrent abuse of alcohol and other substances. With parenteral abuse, the capsule excipients can be expected to result in local tissue necrosis, infection, pulmonary granulomas, and increased risk of endocarditis and valvular heart injury. Parenteral drug abuse is commonly associated with transmission of infectious diseases such as hepatitis and HIV.
Use in Drug and Alcohol Addiction
Tramadol Hydrochloride Extended-Release is an opioid with no approved use for the management of addictive disorders. Its proper usage in individuals with drug or alcohol dependence, either active or in remission is for the management of pain requiring opioid analgesia. Concerns about abuse and addiction should not prevent the proper management of pain. However all patients treated with opioids require careful monitoring for signs of abuse and addiction, because use of opioid analgesic products carries the risk of addiction even under appropriate medical use.
9.3 Dependence
Tolerance is the need for increasing doses of opioids to maintain a defined effect such as analgesia (in the absence of disease progression or other external factors). Physical dependence is manifested by withdrawal symptoms after abrupt discontinuation of a drug or upon administration of an antagonist.
Withdrawal Symptoms
The opioid abstinence or withdrawal syndrome is characterized by some or all of the following: restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia, and mydriasis. Other symptoms also may develop, including irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate.
Generally, tolerance and/or withdrawal are more likely to occur the longer a patient is on continuous opioid therapy.
Withdrawal symptoms may occur if Tramadol Hydrochloride Extended-Release is discontinued abruptly. Onset of adverse events are likely to occur after treatment is stopped. These symptoms may include: anxiety, sweating, insomnia, rigors, pain, nausea, tremors, diarrhea, upper respiratory symptoms, piloerection, and rarely hallucinations. Clinical experiences with tramadol suggests that withdrawal symptoms may be reduced by tapering Tramadol Hydrochloride Extended-Release when discontinuing tramadol therapy [ see DOSAGE AND ADMINISTRATION ( 2) and WARNINGS AND PRECAUTIONS ( 5.10) ].
-
10 OVERDOSAGE
10.1 Human Experience
Acute overdosage with tramadol can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, bradycardia, hypotension, and death.
Deaths due to overdose have been reported with abuse and misuse of tramadol, by ingesting, inhaling, or injecting the crushed tablets. Review of case reports has indicated that the risk of fatal overdose is further increased when tramadol is abused concurrently with alcohol or other CNS depressants, including other opioids.
10.2 Management of Overdose
In the treatment of tramadol overdosage, primary attention should be given to the reestablishment of a patent airway and institution of assisted or controlled ventilation. Supportive measures (including oxygen and vasopressors) should be employed in the management of circulatory shock and pulmonary edema accompanying overdose as indicated. Cardiac arrest or arrhythmias may require cardiac massage or defibrillation.
While naloxone will reverse some, but not all, symptoms caused by overdosage with tramadol, the risk of seizures is also increased with naloxone administration. In animals, convulsions following the administration of toxic doses of Tramadol Hydrochloride Extended-Release could be suppressed with barbiturates or benzodiazepines but were increased with naloxone. Naloxone administration did not change the lethality of an overdose in mice. Hemodialysis is not expected to be helpful in an overdose because it removes less than 7% of the administered dose in a 4-hour dialysis period.
-
11 DESCRIPTION
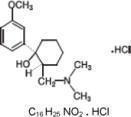
Tramadol Hydrochloride Extended-Release (tramadol hydrochloride) capsules is a centrally acting synthetic analgesic in an extended-release oral formulation. The chemical name for tramadol hydrochloride is (±) cis-2-[(dimethylamino)methyl]-1-(3-methoxyphenyl) cyclohexanol hydrochloride. Its structural formula is:
Figure 1
The molecular weight of tramadol hydrochloride is 299.8. It is a white, bitter, crystalline and odorless powder that is readily soluble in water and ethanol and has a pKa of 9.41. The n-octanol/water log partition coefficient (logP) is 1.35 at pH 7. Tramadol Hydrochloride Extended-Release capsules contain a total dose of tramadol HCl 100, 200 and 300 mg in a combination of immediate-release and extended-release components.
Dosage Immediate-release Extended-release 150 mg 37.5 mg 112.5 mg Tramadol Hydrochloride Extended-Release capsules are white in color. Inactive ingredients include gelatin, titanium dioxide, shellac, yellow iron oxide, lactose monohydrate 200 mesh, microcrystalline cellulose, povidone K30, corn starch, sodium starch glycolate, magnesium stearate, sucrose stearate, hypromellose, talc, polysorbate 80, Eudragit NE 30D, and simethicone emulsion.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Tramadol Hydrochloride Extended-Release contains tramadol, a centrally acting synthetic opioid analgesic. Although its mode of action is not completely understood, from animal tests, at least two complementary mechanisms appear applicable: binding of parent and M1 metabolite to μ-opioid receptors and weak inhibition of reuptake of norepinephrine and serotonin.
Opioid activity is due to both low affinity binding of the parent compound and higher affinity binding of the O-demethylated metabolite M1 to μ-opioid receptors. In animal models, M1 is up to 6 times more potent than tramadol in producing analgesia and 200 times more potent in μ-opioid binding. Tramadol-induced analgesia is only partially antagonized by the opiate antagonist naloxone in several animal tests. The relative contribution of both tramadol and M1 to human analgesia is dependent upon the plasma concentrations of each compound.
12.2 Pharmacodynamics
Tramadol has been shown to inhibit reuptake of norepinephrine and serotonin in vitro, as have some other opioid analgesics. These mechanisms may contribute independently to the overall analgesic profile of tramadol. The relationship between exposure of tramadol and M1 and efficacy has not been evaluated in clinical studies.
Apart from analgesia, tramadol administration may produce a constellation of symptoms (including dizziness, somnolence, nausea, constipation, sweating and pruritus) similar to that of other opioids. In contrast to morphine, tramadol has not been shown to cause histamine release. At therapeutic doses, tramadol has no effect on heart rate, left ventricular function or cardiac index. Orthostatic hypotension has been observed.
12.3 Pharmacokinetics
The analgesic activity of tramadol is due to both parent drug and the M1 metabolite. Tramadol Hydrochloride Extended-Release is administered as a racemate and both tramadol and M1 are detected in the circulation. The C max and AUC of Tramadol Hydrochloride Extended-Release capsules have been observed to be dose-proportional over an oral dose range of 100 to 300 mg in healthy subjects.
Absorption:
After a single dose administration of Tramadol Hydrochloride Extended-Release, T max occurs around 10-12 hours.
The mean C max and AUC of Tramadol Hydrochloride Extended-Release capsules after a 300 mg single dose was 308 ng/mL and 6777 ng*hr/mL, respectively under fasting conditions. Tramadol Hydrochloride Extended-Release is bioequivalent to a reference extended-release tramadol product following a single 300 mg dose under fasting conditions.
At steady-state, Tramadol Hydrochloride Extended-Release at 200 mg has been observed to be bioequivalent to a reference extended-release tramadol product at 200 mg under fasting conditions ( Table 2). Following administration of Tramadol Hydrochloride Extended-Release 200 mg capsules, steady-state plasma concentrations of both tramadol and M1 are achieved within four days of once daily dosing.
Table 2. AUC 0-24: Area Under the Curve in a 24-hour dosing interval
C max: Peak Concentration in a 24-hour dosing interval
C min: Trough Concentration in a 24-hour dosing interval
T max: Time to Peak Concentration
Mean (%CV) Steady-State Pharmacokinetic Parameter Values (N= 38)
Tramadol O-Desmethyl-Tramadol
(M1 Metabolite)Parameter Tramadol hydrochloride Extended Release Capsules 200 mg A Reference Extended-Release Tramadol Product 200 mg Tramadol hydrochloride Extended Release Capsules 200 mg A Reference Extended-Release Tramadol Product
200 mgAUC 0-24 (ng.h/mL) 5678 (27%) 5563 (32%) 1319 (34%) 1302 (40%) C max (ng/mL) 332 (25%) 350 (31%) 70 (34%) 74 (41%) C min
(ng/mL)128 (39%) 125 (45%) 35 (34%) 33 (42%) T max 5.9 (66%) 10 (30%) 11 (37%) 13 (29%) % Fluctuation 88 (19%) 101 (30%) 64 (22%) 76 (30%) Food Effects:
The rate and extent of absorption of Tramadol Hydrochloride Extended-Release capsules (300 mg) are similar following oral administration with or without food. Therefore, Tramadol Hydrochloride Extended-Release capsules can be administered without regard to meals.
Distribution:
The volume of distribution of tramadol was 2.6 and 2.9 liters/kg in male and female subjects, respectively, following a 100 mg intravenous tramadol dose. The binding of tramadol to human plasma proteins is approximately 20% and binding also appears to be independent of concentration up to 10 μg/mL. Saturation of plasma protein binding occurs only at concentrations outside the clinically relevant range.
Metabolism:
Tramadol is extensively metabolized after oral administration. The major metabolic pathways appear to be N – (mediated by CYP3A4 and CYP2B6) and O – (mediated by CYP2D6) demethylation and glucuronidation or sulfation in the liver. One metabolite (O-desmethyl tramadol, denoted M1) is pharmacologically active in animal models. Formation of M1 is dependent on CYP2D6 and as such is subject to inhibition and polymorphism, which may affect the therapeutic response [see DRUG INTERACTIONS ( 7)] .
Elimination:
Tramadol is eliminated primarily through metabolism by the liver and the metabolites are eliminated primarily by the kidneys. Approximately 30% of the dose is excreted in the urine as unchanged drug, whereas 60% of the dose is excreted as metabolites. The remainder is excreted either as unidentified or as unextractable metabolites. The mean plasma elimination half-lives of racemic tramadol and racemic M1 after administration of Tramadol Hydrochloride Extended-Release capsules are approximately 10 and 11 hours, respectively.
Renal Impairment
Impaired renal function results in a decreased rate and extent of excretion of tramadol and its active metabolite, M1. The pharmacokinetics of tramadol was studied in patients with mild or moderate renal impairment after receiving multiple doses of an extended-release tramadol product at 100 mg. There is no consistent trend observed for tramadol exposure related to renal function in patients with mild (CL cr: 50-80 mL/min) or moderate (CL cr: 30-50 mL/min) renal impairment in comparison to patients with normal renal function (CL cr > 80 mL/min). However, exposure of M1 increased 20-40% with increased severity of the renal impairment (from normal to mild and moderate). The pharmacokinetics of tramadol has not been studied in patients with severe renal impairment (CL cr < 30 mL/min). The limited availability of dose strengths of Tramadol Hydrochloride Extended-Release does not permit the dosing flexibility required for safe use in patients with severe renal impairment. Therefore, Tramadol Hydrochloride Extended-Release should not be used in patients with severe renal impairment [see DOSAGE AND ADMINISTRATION ( 2.3), WARNINGS AND PRECAUTIONS (5.14) and USE IN SPECIFIC POPULATIONS ( 8.6)]. The total amount of tramadol and M1 removed during a 4-hour dialysis period is less than 7% of the administered dose.
Hepatic Impairment
Pharmacokinetics of tramadol was studied in patients with mild or moderate hepatic impairment after receiving multiple doses of an extended-release tramadol product at 100 mg. The exposure of (+)- and (-)-tramadol was similar in mild and moderate hepatic impairment patients in comparison to patients with normal hepatic function. However, exposure of (+)- and (-)-M1 decreased ~50% with increased severity of the hepatic impairment (from normal to mild and moderate). The pharmacokinetics of tramadol has not been studied in patients with severe hepatic impairment. After the administration of tramadol immediate-release tablets to patients with advanced cirrhosis of the liver, tramadol area under the plasma concentration time curve was larger and the tramadol and M1 half-lives were longer than subjects with normal hepatic function. The limited availability of dose strengths of Tramadol Hydrochloride Extended-Release does not permit the dosing flexibility required for safe use in patients with severe hepatic impairment. Therefore, Tramadol Hydrochloride Extended-Release should not be used in patients with severe hepatic impairment [see DOSAGE AND ADMINISTRATION ( 2.3), WARNINGS AND PRECAUTIONS (5.14), and USE IN SPECIFIC POPULATIONS ( 8.7)].
Gender
Based on pooled multiple-dose pharmacokinetics studies for an extended-release tramadol product in 166 healthy subjects (111 males and 55 females), the dose-normalized AUC values for tramadol were somewhat higher in females than in males. There was a considerable degree of overlap in values between male and female groups. Dosage adjustment based on gender is not recommended.
Age
The effect of age on pharmacokinetics of Tramadol Hydrochloride Extended-Release has not been studied. Healthy elderly subjects aged 65 to 75 years administered an immediate-release formulation of tramadol, have plasma concentrations and elimination half-lives comparable to those observed in healthy subjects less than 65 years of age. In subjects over 75 years, mean maximum plasma concentrations are elevated (208 vs. 162 ng/mL) and the mean elimination half-life is prolonged (7 vs. 6 hours) compared to subjects 65 to 75 years of age. Adjustment of the daily dose is recommended for patients older than 75 years [see DOSAGE AND ADMINISTRATION ( 2.3)].
Poor / Extensive Metabolizers, CYP2D6
The formation of the active metabolite, M1, is mediated by CYP2D6, a polymorphic enzyme. Approximately 7% of the population has reduced activity of the CYP2D6 isoenzyme of cytochrome P-450 metabolizing enzyme system. These individuals are “poor metabolizers” of debrisoquine, dextromethorphan and tricyclic antidepressants, among other drugs. Based on a population PK analysis of Phase 1 studies with IR tablets in healthy subjects, concentrations of tramadol were approximately 20% higher in "poor metabolizers" versus "extensive metabolizers," while M1 concentrations were 40% lower.
CYP2D6 Inhibitors
In vitro drug interaction studies in human liver microsomes indicate that concomitant administration with inhibitors of CYP2D6 such as fluoxetine, paroxetine, and amitriptyline could result in some inhibition of the metabolism of tramadol.
Quinidine
Tramadol is metabolized to active metabolite M1 by CYP2D6. Coadministration of quinidine, a selective inhibitor of CYP2D6, with tramadol ER resulted in a 50-60% increase in tramadol exposure and a 50-60% decrease in M1 exposure. The clinical consequences of these findings are unknown.
To evaluate the effect of tramadol, a CYP2D6 substrate on quinidine, an in vitro drug interaction study in human liver microsomes was conducted. The results from this study indicate that tramadol has no effect on quinidine metabolism.
[see WARNINGS AND PRECAUTIONS ( 5.1, 5.3) and DRUG INTERACTIONS ( 7.3) ].
CYP3A4 Inhibitors and Inducers
Since tramadol is also metabolized by CYP3A4, administration of CYP3A4 inhibitors, such as ketoconazole and erythromycin, or CYP3A4 inducers, such as rifampin and St. John's Wort, with Tramadol Hydrochloride Extended-Release may affect the metabolism of tramadol leading to altered tramadol exposure [see WARNINGS AND PRECAUTIONS ( 5.1, 5.3 ) and DRUG INTERACTIONS ( 7.3) ].
Cimetidine
Concomitant administration of tramadol immediate-release tablets with cimetidine, a weak CPY3A4 inhibitor, does not result in clinically significant changes in tramadol pharmacokinetics. No alteration of the Tramadol Hydrochloride Extended-Release dosage regimen with cimetidine is recommended.
-
13 NON-CLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity assessment has been conducted in mice, rats and p53(+/-) heterozygous mice. A slight, but statistically significant, increase in two common murine tumors, pulmonary and hepatic, was observed in a mouse carcinogenicity study, particularly in aged mice. Mice were dosed orally up to 30 mg/kg (90 mg/m 2 or 0.5 times the maximum daily human dosage of 185 mg/m 2) for approximately two years, although the study was not done with the Maximum Tolerated Dose. This finding is not believed to suggest risk in humans.
No treatment-related tumors were noted in a rat carcinogenicity study (dosing orally up to 30 mg/kg, 180 mg/m 2, or equivalent to the maximum daily human dosage) or in a second study where rats were treated with up to 75 mg/kg/day for males and 100 mg/kg/day for females (approximately 2.4 and 3.2-fold MDHD, respectively) for two years. However, the excessive decrease in body weight gain observed in the rat study might have reduced their sensitivity to any potential carcinogenic effect of the drug.
No carcinogenic effect of tramadol was observed in p53(+/–)-heterozygous mice at oral doses up to 150 mg/kg/day (approximately 2.4-fold maximum daily human dose [MDHD] of 300 mg/day for a 60 kg adult based on body surface conversion) for 26 weeks.
Tramadol was not mutagenic in the following assays: a bacterial reverse mutation assay using Salmonella and E. coli, a mouse lymphoma assay (in the absence of metabolic activation), chromosomal aberration test in Chinese hamsters, a bone marrow micronucleus test in mice and Chinese hamsters, and a dominant lethal mutation test in mice. Mutagenic results occurred in the presence of metabolic activation in the mouse lymphoma assay and micronucleus test in rats. Overall, the weight of evidence from these tests indicates that tramadol does not pose a genotoxic risk to humans.
No effects on fertility were observed for tramadol at oral dose levels up to 50 mg/kg/day in male and female rats (1.6-fold the MDHD).
-
14 CLINICAL STUDIES
Tramadol Hydrochloride Extended-Release is bioequivalent under fasting conditions to another extended-release tramadol product [see CLINICAL PHARMACOLOGY ( 12.3)] which did demonstrate efficacy in two of four clinical trials of patients with chronic pain. To qualify for inclusion into these studies, patients were required to have moderate to moderately severe pain as defined by a pain intensity score of ≥40 mm, off previous medications, on a 0 – 100 mm visual analog scale (VAS).
In one 12-week randomized, double-blind, placebo-controlled study, patients with moderate to moderately severe pain due to osteoarthritis of the knee and/or hip were administered doses from 100 mg to 400 mg daily. Treatment with the extended-release tramadol product was initiated at 100 mg once daily for four days then increased by 100 mg per day increments every five days to the randomized fixed dose. Between 51% and 59% of patients in active treatment groups completed the study and 56% of patients in the placebo group completed the study. Discontinuations due to adverse events were more common in the extended-release tramadol product 200 mg, 300 mg and 400 mg treatment groups (20%, 27%, and 30% of discontinuations, respectively) compared to 14% of the patients treated with the extended-release tramadol product 100 mg and 10% of patients treated with placebo.
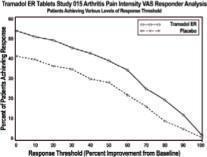
Pain, as assessed by the WOMAC Pain subscale, was measured at 1, 2, 3, 6, 9, and 12 weeks and change from baseline assessed. A responder analysis based on the percent change in WOMAC Pain subscale demonstrated a statistically significant improvement in pain for the 100 mg and 200 mg treatment groups compared to placebo (see Figure 2).
Figure 2
In one 12-week randomized, double-blind, placebo-controlled flexible-dosing trial of the extended-release tramadol product in patients with osteoarthritis of the knee, patients titrated to an average daily dose of approximately 270 mg/day. Forty-nine percent of patients randomized to the active treatment group completed the study, while 52% of patients randomized to placebo completed the study. Most of the early discontinuations in the active treatment group were due to adverse events, accounting for 27% of the early discontinuations in contrast to 7% of the discontinuations from the placebo group. Thirty-seven percent of the placebo-treated patients discontinued the study due to lack of efficacy compared to 15% of active-treated patients. The active treatment group demonstrated a statistically significant decrease in the mean Visual Analog Scale (VAS) score, and a statistically significant difference in the responder rate, based on the percent change from baseline in the VAS score, measured at 1, 2, 4, 8, and 12 weeks, between patients receiving the extended-release tramadol product and placebo (see Figure 3).
Figure 3
Four randomized, placebo-controlled clinical trials of Tramadol Hydrochloride Extended-Release were conducted, none of which demonstrated efficacy but which differed in design from the preceding clinical studies described. Two trials were 12-week randomized placebo-controlled trials of Tramadol Hydrochloride Extended-Release 100 mg/day, 200 mg/day, and 300 mg/day versus placebo in patients with moderate to moderately severe osteoarthritis pain of the hip and knee. The other two 12 week trials were similar in design, but only studied Tramadol Hydrochloride Extended-Release 300 mg/day. In this fixed-dose design, subjects were required to titrate to a fixed dose, even if their pain responded to a lower titration dose.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Tramadol Hydrochloride Extended-Release capsules are supplied as opaque white hard gelatin capsules, imprinted as follows.
150 mg Capsules: White capsule imprinted with gold ink “ G 322” on cap and “ 150” between lines on the body
Repackaged by:
Medsource Pharmaceuticals
Rancho Santa Margarita, CA 92688
-
17 PATIENT COUNSELING INFORMATION
Inform patients that:
- Tramadol Hydrochloride Extended-Release is for oral use only and should be swallowed whole. The capsule should not be chewed, dissolved, crushed or split.
- Tramadol Hydrochloride Extended-Release may cause seizures and/or serotonin syndrome with concomitant use of serotonergic agents (including SRIs, NRIs, and triptans) or drugs that significantly reduce the metabolic clearance of tramadol.
- Not to change the prescribed single-dose or 24-hour dosing regimen of Tramadol Hydrochloride Extended-Release, and that exceeding the prescribed dose can result in respiratory depression, seizures or death.
- Tramadol Hydrochloride Extended-Release may impair mental or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery.
- Tramadol Hydrochloride Extended-Release should not be taken with alcohol containing beverages.
- Tramadol Hydrochloride Extended-Release should be used with caution when taking medications such as tranquilizers, hypnotics or other opiate containing analgesics.
- Instruct female patients to inform the prescriber if they are pregnant, think they might become pregnant, or are trying to become pregnant.
- Tramadol Hydrochloride Extended-Release is to be taken once-a-day and at approximately the same time every day. Also, exceeding these recommendations and the maximum recommended daily dose can result in respiratory depression, seizures or death.
- Elderly patients, especially those over 75 years of age, and other patients who have renal or hepatic impairments may need to be cautioned about reduced dosages.
- Not to abruptly withdraw or discontinue tramadol therapy, as clinical experience with tramadol suggests the possible onset of signs and symptoms of withdrawal. These affects may be reduced by tapering tramadol therapy.
- Tramadol Hydrochloride Extended-Release must be kept out of reach of children.
Revised: 06/2011
- Principal Display Panel - 60 count
-
INGREDIENTS AND APPEARANCE
TRAMADOL HYDROCHLORIDE
tramadol hydrochloride capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 45865-709(NDC: 76218-0708) Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRAMADOL HYDROCHLORIDE (UNII: 9N7R477WCK) (TRAMADOL - UNII:39J1LGJ30J) TRAMADOL HYDROCHLORIDE 150 mg Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SHELLAC (UNII: 46N107B71O) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SUCROSE STEARATE (UNII: 274KW0O50M) POVIDONE K30 (UNII: U725QWY32X) STARCH, CORN (UNII: O8232NY3SJ) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) TALC (UNII: 7SEV7J4R1U) POLYSORBATE 80 (UNII: 6OZP39ZG8H) ETHYL ACRYLATE AND METHYL METHACRYLATE COPOLYMER (2:1; 750000 MW) (UNII: P2OM2Q86BI) Product Characteristics Color white (White with Gold Ink) Score no score Shape CAPSULE Size 19mm Flavor Imprint Code G;322;150 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 45865-709-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 07/27/2015 2 NDC: 45865-709-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 07/27/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022370 03/14/2012 Labeler - Medsource Pharmaceuticals (833685915) Registrant - Medsource Pharmaceuticals (833685915) Establishment Name Address ID/FEI Business Operations Medsource Pharmaceuticals 833685915 repack(45865-709)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.