FUNGIFREE Nail Repair Solution
FUNGIFREE Nail Repair Solution by
Drug Labeling and Warnings
FUNGIFREE Nail Repair Solution by is a Otc medication manufactured, distributed, or labeled by YITONGBADA (SHENZHEN) INTERNATIONAL TRADE CO., LTD. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
FUNGIFREE NAIL REPAIR SOLUTION- tolnaftate ointment
YITONGBADA (SHENZHEN) INTERNATIONAL TRADE CO., LTD
----------
FUNGIFREE Nail Repair Solution
Drug Facts
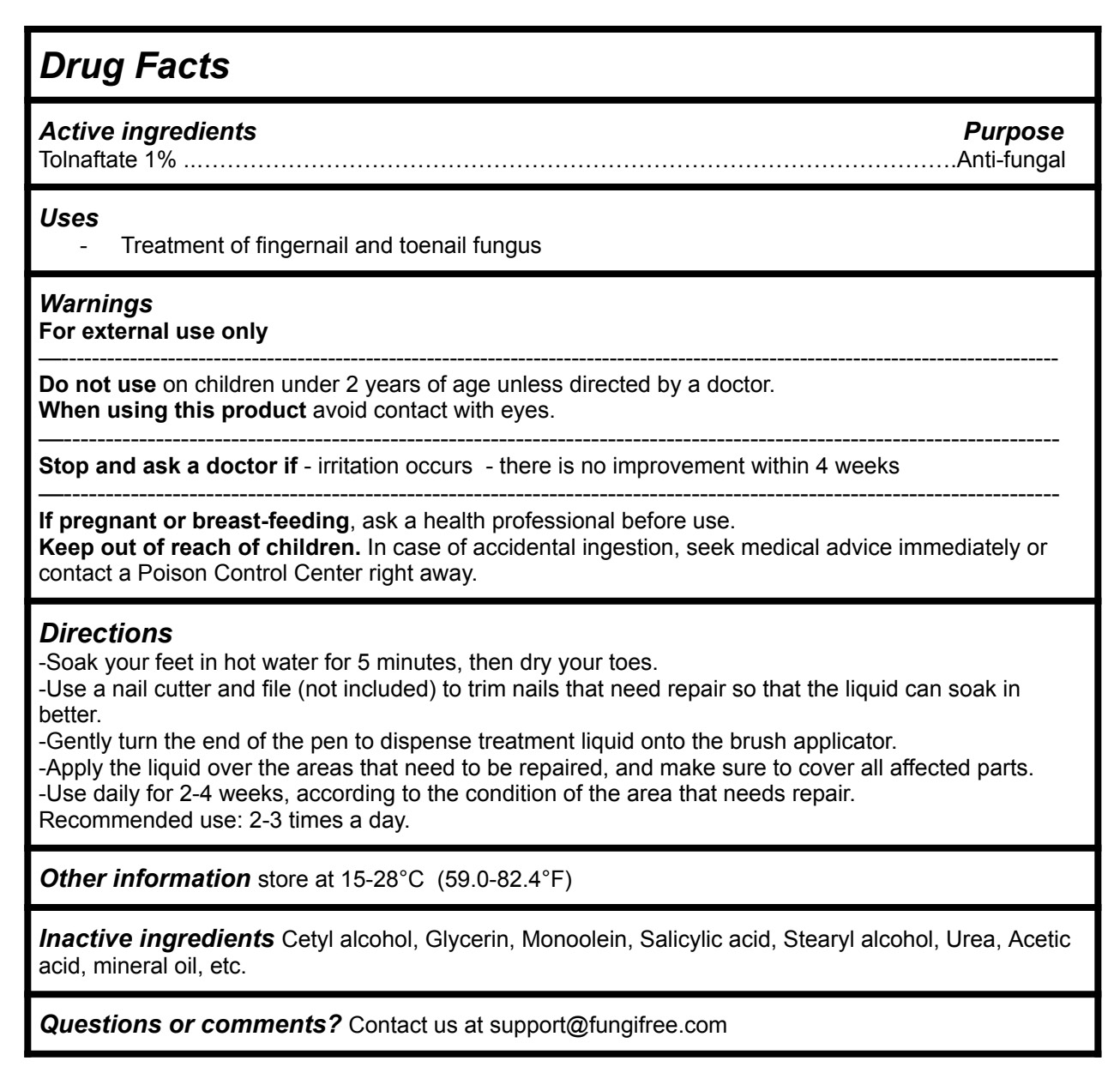
Directions
-Soak your feet in hot water for 5 minutes, then dry your toes.
-Use a nail cutter and file (not included) to trim nails that need repair so that the liquid can soak in better.
-Gently turn the end of the pen to dispense treatment liquid onto the brush applicator.
-Apply the liquid over the areas that need to be repaired, and make sure to cover all affected parts.
-Use daily for 2-4 weeks, according to the condition of the area that needs repair.
Recommended use: 2-3 times a day.
| FUNGIFREE NAIL REPAIR SOLUTION
tolnaftate ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - YITONGBADA (SHENZHEN) INTERNATIONAL TRADE CO., LTD (725220463) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.